SOLANA BEACH, CALIFORNIA / ACCESS Newswire / October 1, 2025 / ClearPoint Neuro, Inc. (Nasdaq:CLPT) (the "Company"), a global device, cell, and gene therapy-enabling company offering precise navigation to the brain and spine, today formally announced the development and demonstration of the Company's proprietary Robotic Neuro-Navigation System. This new product category will enable the ClearPoint Neuro Navigation software to operate the KUKA LBR Med Robotic Arm to support all minimally invasive cranial surgical procedures including cell and gene therapy infusions, laser catheter placement, biopsy workflows and deep brain stimulation and stereotactic EEG lead placements. The Company will demonstrate a prototype of this robotic system at the 75th Annual Congress of Neurological Surgeons (CNS) in Los Angeles October 13th-15th.
"Everything we do at ClearPoint Neuro is viewed through the lens of 'how does this help us extend our lead in neuro drug delivery and help our partners prepare for the commercialization of these new cell and gene therapies," commented Jeremy Stigall, CBO and GM of the Biologics and Drug Delivery Business at ClearPoint. "Many of our BioPharma partners have the same dilemma that ClearPoint is helping to solve. On one side, they see the clear value of MRI guidance for drug delivery clinical trials and regulatory submissions, yet the requirements for MRI guidance can be viewed as narrow in flexibility and surgeon choice and can, therefore, limit adoption. On the other side, BioPharma understands that providing too much flexibility with the surgical technique can lead to less-than-optimal results because the ability to train on the crucial surgical technique in a consistent and organized manner can be lost with that flexibility. Just like a lean manufacturing line, you cannot increase quality and reduce procedure time and cost without consistency and repetition. BioPharma needs a single navigation partner that can provide that sweet spot of offering BOTH consistency and flexibility."
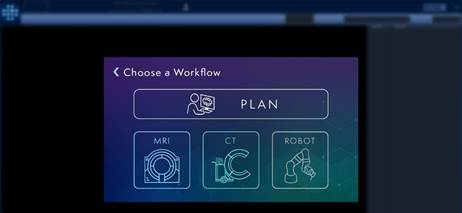 Figure 1: Prototype ClearPoint software launch screen highlighting comprehensive pre-procedure planning followed by physician choice on plan deployment workflow
Figure 1: Prototype ClearPoint software launch screen highlighting comprehensive pre-procedure planning followed by physician choice on plan deployment workflow
"The addition of the ClearPoint Neuro Robotic Neuro-Navigation System to our portfolio is designed to do exactly that - to provide consistency and flexibility to the commercial launches of various cell and gene therapies," continued Stigall. "First, for consistency, there will be one ClearPoint software planning module which is already used today. This software will be the first step for the surgical team to plan every patient's drug infusion plan, often days before the surgery itself. Second, the surgical team will then decide through which hardware mechanism to execute that surgical plan across three different ClearPoint options: the traditional MRI guided technique, the new ClearPoint 3.0 iCT guided technique in the operating room, and the future ClearPoint Robotic assisted technique also using iCT. Each of these options benefit from the same ClearPoint software workflow, and many advanced drug delivery features. We believe this strategy is unique, and that ClearPoint is best suited to take on this responsibility with our drug delivery portfolio, including co-labeled cannula-based routes-of-administration, pre-clinical CRO testing and live specialist procedural support."
"We have reached a point in medicine that if you close your eyes and imagine the future of neurosurgery, there is no way that robotics are not a significant part of that future," commented Joe Burnett, President and CEO of ClearPoint Neuro. "We have been very thoughtful and deliberate in our approach to enter the neuro robotics market. In years past, a company would have to develop a complete robotic system from scratch and bear the entire development risk and significant costs along the way. Today, by working with KUKA, we can leverage their decades of experience which has produced an FDA cleared and CE marked robotic arm and combine that with the decades of experience we have at ClearPoint, creating a state-of-the-art neuro navigation software to drive the robot in the same 3D space. This dramatically decreases the development cost and accelerates our speed to market. The timing is crucial as we are helping our BioPharma partners to identify regional treatment centers and to add surgical capacity in preparation of more significant commercial case volume in the next couple of years."
The ClearPoint Neuro Robotic Neuro-Navigation System has not yet been submitted to any regulatory body. The Company will continue product development with the goal of commercialization in concert with the approval of multiple neuro cell and gene therapies which are currently under FDA expedited review.
About ClearPoint Neuro
ClearPoint Neuro is a device, cell, and gene therapy-enabling company offering precise navigation to the brain and spine. The Company uniquely provides both established clinical products as well as preclinical development services for controlled drug and device delivery. The Company's flagship product, the ClearPoint Neuro Navigation System, has FDA clearance and is CE-marked. ClearPoint Neuro is engaged with healthcare and research centers in North America, Europe, Asia, and South America. The Company is also partnered with the most innovative pharmaceutical/biotech companies, academic centers, and contract research organizations, providing solutions for direct central nervous system delivery of therapeutics in preclinical studies and clinical trials worldwide. To date, thousands of procedures have been performed and supported by the Company's field-based clinical specialist team, which offers support and services to our customers and partners worldwide. For more information, please visit www.clearpointneuro.com.
Forward-Looking Statements
This press release contains forward-looking statements within the context of the federal securities laws, including the Company's product development plans, and the expectation for commercial objectives, potential benefits, and timing of a product that remains in the initial stage of development. These forward-looking statements are based on management's current expectations and are subject to the risks inherent in the business, which may cause the Company's actual results to differ materially from those expressed in or implied by forward-looking statements. Particular uncertainties and risks include those relating to: macroeconomic and inflationary conditions; regulatory and policy uncertainty; the introduction of or changes in tariffs, sanctions, or trade barriers; changes in monetary policy; geopolitical trends, such as protectionism and economic nationalism; future revenue from sales of the Company's products and services; the Company's ability to market, commercialize and achieve broader market acceptance for new products and services offered by the Company; the ability of our biologics and drug delivery partners to achieve commercial success, including their use of the Company's products and services in their delivery of therapies; the Company's ability to maintain its current relationships with biologics and drug delivery partners or enter into new relationships with such partners; the Company's expectations, projections and estimates regarding expenses, future revenue, capital requirements, and the availability of and the need for additional financing; the Company's ability to obtain additional funding to support its research and development programs; the ability of the Company to manage the growth of its business; the Company's ability to attract and retain its key employees; and risks inherent in the research, development, and regulatory approval of the Company's new products, including the ability to obtain and maintain regulatory clearances; manufacturing scale-up; supply chain and quality system readiness; intellectual property protection and freedom to operate; market acceptance and competitive dynamics. More detailed information on these and additional factors that could affect the Company's actual results are described in the "Risk Factors" section of the Company's Annual Report on Form 10-K for the year ended December 31, 2024, and the Company's Quarterly Report on Form 10-Q for the three months ended June 30, 2025, both of which have been filed with the Securities and Exchange Commission. The Company does not assume any obligation to update these forward-looking statements.
Contact:
Investor Relations:
Danilo D'Alessandro, Chief Financial Officer
(888) 287-9109 ext. 3
This email address is being protected from spambots. You need JavaScript enabled to view it.
| Last Trade: | US$13.51 |
| Daily Change: | -0.48 -3.43 |
| Daily Volume: | 891,140 |
| Market Cap: | US$383.950M |
November 20, 2025 November 06, 2025 November 06, 2025 November 05, 2025 October 07, 2025 | |

ClearPoint Neuro is a global therapy-enabling platform company providing stereotactic navigation and delivery to the brain. Applications of our ClearPoint Neuro Navigation System include electrode lead placement, placement of catheters, and biopsy. The platform has FDA clearance...
CLICK TO LEARN MORE
ClearPoint Neuro is a global therapy-enabling platform company providing stereotactic navigation and delivery to the brain. Applications of our ClearPoint Neuro Navigation System include electrode lead placement, placement of catheters, and biopsy. The platform has FDA clearance...
CLICK TO LEARN MOREEnd of content
No more pages to load