CAMBRIDGE, Mass., Jan. 07, 2025 (GLOBE NEWSWIRE) -- Immuneering Corporation (Nasdaq: IMRX), a clinical-stage oncology company seeking to develop and commercialize more effective and better tolerated therapies for cancer patients, today announced a positive data update from three pancreatic cancer arms of its ongoing Phase 2a trial of lead program IMM-1-104, as well as plans to expand the Phase 2a trial to include three additional combination arms. While approved MEK inhibitors mainly benefit a subset of patients with BRAF-driven tumors, IMM-1-104 was designed to improve tolerability and expand indications to include RAS-mutated tumors such as those found in most pancreatic cancers.
“We are excited to report an updated ORR of 43% and DCR of 86% for IMM-1-104 in combination with modified gemcitabine/nab-paclitaxel in first-line pancreatic cancer patients. For reference, the benchmark reported for gemcitabine/nab-paclitaxel in this setting had an ORR of 23% and DCR of 48%. We look forward to reporting further data in the second quarter of 2025 and have started planning for a potential pivotal clinical trial,” said Ben Zeskind, Ph.D., CEO of Immuneering.
Dr. Zeskind continued: “Today we are also sharing initial data from IMM-1-104 in combination with modified FOLFIRINOX in first-line pancreatic cancer patients. We observed target lesion shrinkage across all evaluable patients, including a 100% reduction, a rare event in this patient population. Additionally, we are reporting initial data from our monotherapy arm of IMM-1-104 in second-line pancreatic cancer. We saw clear activity, including a partial response with a 67% target lesion reduction. We believe these results provide substantiating evidence of IMM-1-104’s contribution in combination with current therapies.”
Dr. Zeskind concluded: “Importantly, we continue to observe a highly differentiated safety profile for IMM-1-104, which we designed to be better tolerated and more active than existing approved MEK inhibitors already driving annual net sales of ~$2.4 billion in 2023. Accordingly, we plan to add three new Phase 2a combination arms: IMM-1-104 with a BRAF inhibitor in BRAF-mutant melanoma, and IMM-1-104 with an immune checkpoint inhibitor in both melanoma and NSCLC. We expect to initiate these arms in 2025. Today we are setting a path to break new ground in indications where no MEK inhibitors have been approved, including pancreatic cancer, and aim to provide a better tolerated and more effective alternative where MEK inhibitors are already helping patients.”
Updated Data from Phase 2a Arm Evaluating IMM-1-104 with Modified Gemcitabine/nab-Paclitaxel in First Line Pancreatic Cancer as of December 5, 2024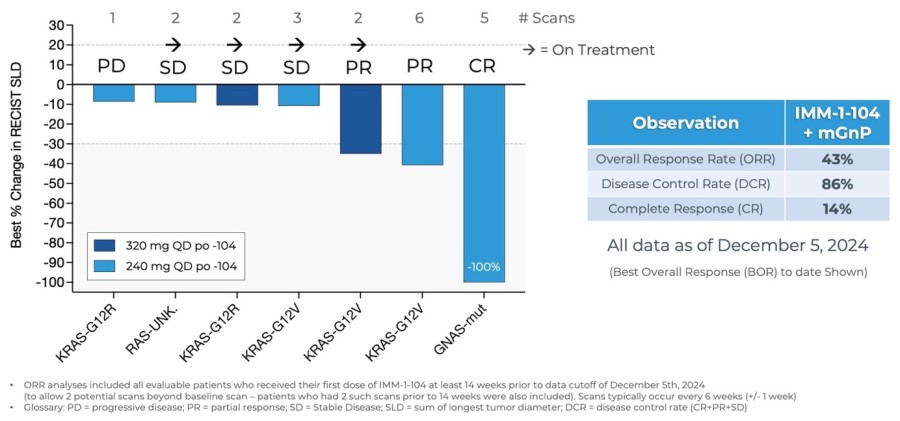
[1] Von Hoff, et al. N Engl J Med 2013;369:1691-1703, [2] Ahn DH, et al. Therapeutic Advances in Medical Oncology. 2017;9(2):75-82
“Immuneering’s Phase 2a data in first-line pancreatic cancer are very promising,” said Tanios Bekaii-Saab, M.D., Leader of the Gastrointestinal Cancer Disease Group for the Mayo Clinic Cancer Center enterprise-wide and Medical Oncology consultant in Mayo Clinic in Phoenix, Arizona. “If current trends continue, the combination of IMM-1-104 with modified gemcitabine/nab-paclitaxel may provide improved efficacy and tolerability versus gemcitabine/nab-paclitaxel in the first-line pancreatic cancer setting, where patients continue to urgently need better options. In addition, having a MEK inhibitor that appears to be as well-tolerated as IMM-1-104 may provide new opportunities for patients with different types of cancer.”
Initial Data from Phase 2a Arm Evaluating IMM-1-104 with Modified FOLFIRINOX in First Line Pancreatic Cancer as of December 5, 2024
Initial Data from Phase 2a Arm Evaluating IMM-1-104 Monotherapy in Second Line Pancreatic Cancer as of December 5, 2024
“Having demonstrated compelling activity in both the combination and monotherapy settings for pancreatic cancer, the emerging tolerability profile for IMM-1-104 is also highly promising,” said Brett Hall, Ph.D., Chief Scientific Officer, Immuneering Corporation. “Looking at the table of treatment-related adverse events observed in greater than 10% of patients in our monotherapy arm, no Grade 3 or Grade 4 events were observed, and only a handful of Grade 2 events were observed. The maturing safety profile for IMM-1-104 gives us confidence that Immuneering may have developed a better tolerated MEK-inhibitor, with exciting potential for vertical, immune-modifying, and orthogonal combinations. We expect to share expanded development plans for IMM-1-104 beyond pancreatic cancer, as we continue to explore options with investigators and third parties.”
Immuneering previously announced that IMM-1-104 received Fast Track designation from the FDA for the treatment of first- and second-line pancreatic cancer, along with orphan drug designation. The FDA also recently granted Fast Track designation for IMM-1-104 as a treatment for patients with unresectable or metastatic NRAS-mutant melanoma who have progressed on or are intolerant to PD-1/PD-L1 based immune checkpoint inhibitors. Today’s data update follows initial data that was presented in September 2024 on the trial’s arm studying IMM-1-104 in combination with modified gemcitabine/nab-paclitaxel in first-line pancreatic cancer.
Today, Immuneering also announced initial pharmacokinetic, pharmacodynamic and safety data from the Phase 1 portion of the company’s Phase 1/2a trial of IMM-6-415. To date, IMM-6-415 has demonstrated its potential to induce Deep Cyclic Inhibition, and in doing so has been well tolerated – consistent with what was observed preclinically for the development candidate.
Near-Term Milestone Expectations
IMM-1-104
Conference Call
Immuneering will host a conference call and live webcast at 8:30 a.m. ET / 5:30 a.m. PT on January 7, 2025, to discuss the data and provide a business update. Individuals interested in listening to the live conference call may do so by dialing (800) 715-9871 for U.S callers and (646) 307-1963 for other locations and reference conference ID 4497245, or from the webcast link in the “investors” section of the company's website at www.immuneering.com A webcast replay will be available in the investor relations section on the company’s website for 90 days following the completion of the call.
About Immuneering Corporation
Immuneering is a clinical-stage oncology company seeking to develop and commercialize more effective and better tolerated therapies for cancer patients. The Company’s lead product candidate, IMM-1-104, is an oral, once-daily deep cyclic inhibitor of MEK designed to improve tolerability and expand indications to include RAS-driven tumors such as most pancreatic cancers. IMM-1-104 is currently in a Phase 1/2a trial in patients with advanced solid tumors including pancreatic cancer. IMM-6-415 is an oral, twice-daily deep cyclic inhibitor of MEK currently in a Phase 1/2a trial in patients with advanced solid tumors harboring RAS or RAF mutations. The company’s development pipeline also includes several early-stage programs. For more information, please visit www.immuneering.com.
Forward-Looking Statements
This press release contains forward-looking statements, including within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including, without limitation, statements regarding: Immuneering’s plans to develop, manufacture and commercialize its product candidates; the treatment potential of IMM-1-104 and IMM-6-415, alone or in combination with other agents, including chemotherapy, PD-1 inhibitors and BRAF inhibitors; the future sales of approved MEK inhibitors; the plans and objectives of Company management for future operations, including with respect to the planning and execution of additional IMM-1-104 combination trials and potential pivotal trial of IMM-1-104 in combination with modified gemcitabine/nab-paclitaxel; and the timing for release of additional results from the Phase 2a portion of the trial for IMM-1-104.
These forward-looking statements are based on management’s current expectations. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: the risks inherent in oncology drug research and development, including target discovery, target validation, lead compound identification, and lead compound optimization; we have incurred significant losses, are not currently profitable and may never become profitable; our projected cash runway; our need for additional funding and ability to continue as a going concern; our unproven approach to therapeutic intervention; our ability to address regulatory questions and the uncertainties relating to regulatory filings, reviews and approvals; the lengthy, expensive, and uncertain process of clinical drug development, including potential delays in or failure to obtain regulatory approvals; our reliance on third parties and collaborators to conduct our clinical trials, manufacture our product candidates, and develop and commercialize our product candidates, if approved; failure to compete successfully against other drug companies; protection of our proprietary technology and the confidentiality of our trade secrets; potential lawsuits for, or claims of, infringement of third-party intellectual property or challenges to the ownership of our intellectual property; our patents being found invalid or unenforceable; costs and resources of operating as a public company; and unfavorable or no analyst research or reports.
These and other important factors discussed under the caption “Risk Factors” in our Quarterly Report on Form 10-Q for the period ended September 30, 2024, and our other reports filed with the U.S. Securities and Exchange Commission, could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management's estimates as of the date of this press release. While we may elect to update such forward-looking statements at some point in the future, except as required by law, we disclaim any obligation to do so, even if subsequent events cause our views to change. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this press release.
Media Contact:
Gina Nugent
This email address is being protected from spambots. You need JavaScript enabled to view it.
Investor Contact:
Laurence Watts
619-916-7620
This email address is being protected from spambots. You need JavaScript enabled to view it.

| Last Trade: | US$6.40 |
| Daily Change: | -0.29 -4.33 |
| Daily Volume: | 772,891 |
| Market Cap: | US$413.250M |
November 12, 2025 September 24, 2025 | |

Recursion Pharmaceuticals is a clinical stage TechBio company leading the space by decoding biology to industrialize drug discovery. Enabling its mission is the Recursion OS, a platform built across diverse technologies that continuously expands one of the world’s largest....
CLICK TO LEARN MORE
C4 Therapeutics is pioneering a new class of small-molecule drugs that selectively destroy disease-causing proteins via degradation using the innate machinery of the cell. This targeted protein degradation approach offers advantages over traditional drugs, including the potential to treat a wider range of diseases...
CLICK TO LEARN MOREEnd of content
No more pages to load