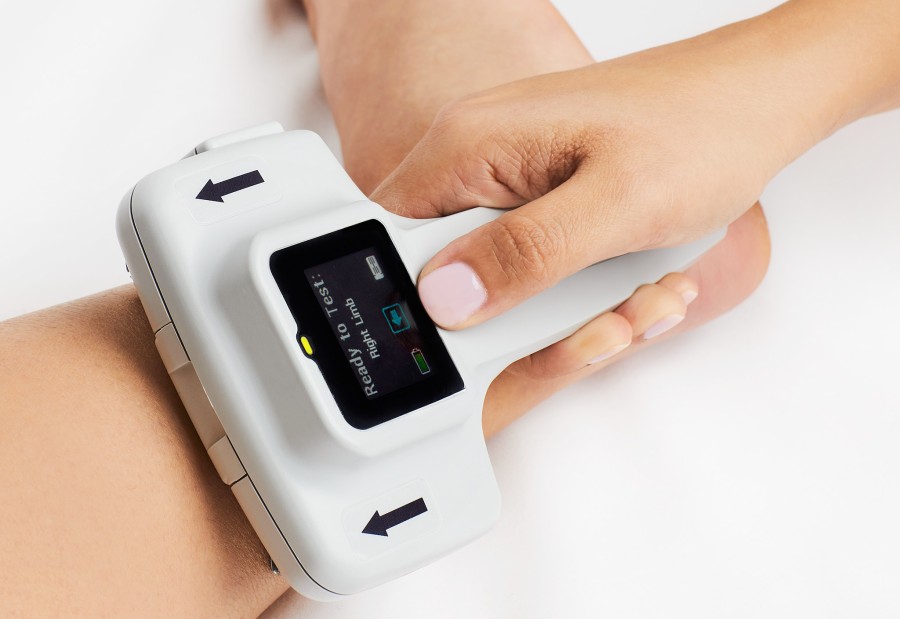
WOBURN, Mass., Jan. 23, 2023 (GLOBE NEWSWIRE) -- NeuroMetrix, Inc. (Nasdaq: NURO) today announced the commercial availability of DPNCheck 2.0, the Company's latest point-of-care device that leverages gold-standard nerve conduction technology to detect peripheral neuropathy. DPNCheck 2.0 provides rapid patient screening with quantitative measurement of peripheral nerve function. It features an easy-to-use touchscreen, onboard step-by-step instructions, improved temperature compensation, real-time nerve response display and other enhancements. Its companion software facilitates clinical documentation of test results. Cloud-based aggregation of population-health data and integration with provider EHR systems is planned for later this year.
Peripheral neuropathy, the systemic degeneration of peripheral nerves, is a common and debilitating condition that may affect up to 30% of the Medicare-aged population. An increasing number of healthcare providers and health plans that support the Medicare population have recently turned their attention to early detection and management of this condition to help reduce their patients’ risk of foot ulcers, falls, pain and other complications. By incorporating screening for peripheral neuropathy using nerve conduction technology, it is possible to uncover elevated risk for these complications earlier and more accurately than traditional clinical approaches such as the monofilament and tuning fork tests.
“For more than a decade, our first-generation DPNCheck device has been used and trusted by thousands of providers to assess over 2-million patients,” said Shai N. Gozani, M.D., Ph.D., Chief Executive Officer, NeuroMetrix. “We are excited to launch our newest version to even better address the needs of our clinical partners. We’ve opened the door to more widespread screening with a user-friendly design that makes it faster and easier for care organizations to roll out and train their point-of-care staff and deploy testing.”
DPNCheck 2.0 will be presented at the Medicare Advantage Leadership Innovations National Conference January 24-25, 2023 at the Renaissance Glendale, AZ. Please visit Neurometrix booth #21 to see the device in action and learn more from NeuroMetrix’s value-based care and population health experts. For more information, visit www.DPNCheck.com.
About NeuroMetrix
NeuroMetrix is an innovation-driven company with a mission to improve individual and population health through novel medical devices and technology solutions for neurological disorders and pain syndromes. The Company has three commercial products. DPNCheck® is a diagnostic device that provides rapid, point-of-care detection of peripheral neuropathies. ADVANCE® is a diagnostic device that provides automated, in-office nerve conduction studies for the evaluation of focal neuropathies. Quell® Fibromyalgia is a wearable neuromodulator that is the first and only FDA-authorized medical device to help reduce the symptoms of fibromyalgia. For more information, visit www.neurometrix.com.
Source: NeuroMetrix, Inc.
Thomas T. Higgins
SVP and Chief Financial Officer
781-314-2761
This email address is being protected from spambots. You need JavaScript enabled to view it.

| Last Trade: | US$4.58 |
| Daily Volume: | 0 |
| Market Cap: | US$9.340M |
November 05, 2024 August 06, 2024 May 15, 2024 March 14, 2024 February 22, 2024 | |

Immix Biopharma is a clinical-stage biopharmaceutical company pioneering a novel class of CAR-T cell therapies and Tissue-Specific Therapeutics targeting oncology and immuno-dysregulated diseases with >75 patients treated to-date. Our lead cell therapy asset is NXC-201...
CLICK TO LEARN MORE
Chimerix is on a mission to develop medicines that meaningfully improve and extend the lives of patients facing deadly diseases. The company is devoted to filling gaps in the treatment paradigm. Chimerix’s most advanced clinical-stage program is in development for H3 K27M-mutant glioma....
CLICK TO LEARN MOREEnd of content
No more pages to load