DENVER, Aug. 27, 2025 /PRNewswire/ -- As part of its 25th-anniversary celebration, DaVita, a leading provider of comprehensive kidney care, is proud to spotlight the groundbreaking work of its wholly owned research arm, DaVita Clinical Research (DCR). For a quarter-century, DCR has played a pivotal role in advancing kidney care — driving access to new therapies, improving clinical outcomes and shaping the future of nephrology through rigorous research and clinical trials.
"Clinical research has revolutionized the way we understand and treat entire disease states, including chronic kidney disease," said Jeff Giullian, MD, chief medical officer for DaVita. "I've seen firsthand how breakthroughs in research — and the contributions of DaVita Clinical Research — have enabled providers to deliver more personalized, effective care that improves patients' lives. This milestone is a reflection of DaVita's enduring commitment to science and education as drivers of a healthier future."
Rapid Response in Times of Change
DCR has consistently adapted to meet the evolving demands of healthcare, contributing to landmark efforts across the industry:
"Research and data-driven insights help fuel innovation, which is the key to continuing to improve quality of care," said Francesca Tentori, MD, MSCI, vice president of outcomes research and patient empowerment for DaVita. "We're proud of the insights we've uncovered over the past 25 years, and we're excited to continue driving industry-leading outcomes research and clinical trials that advance care for individuals with kidney disease."
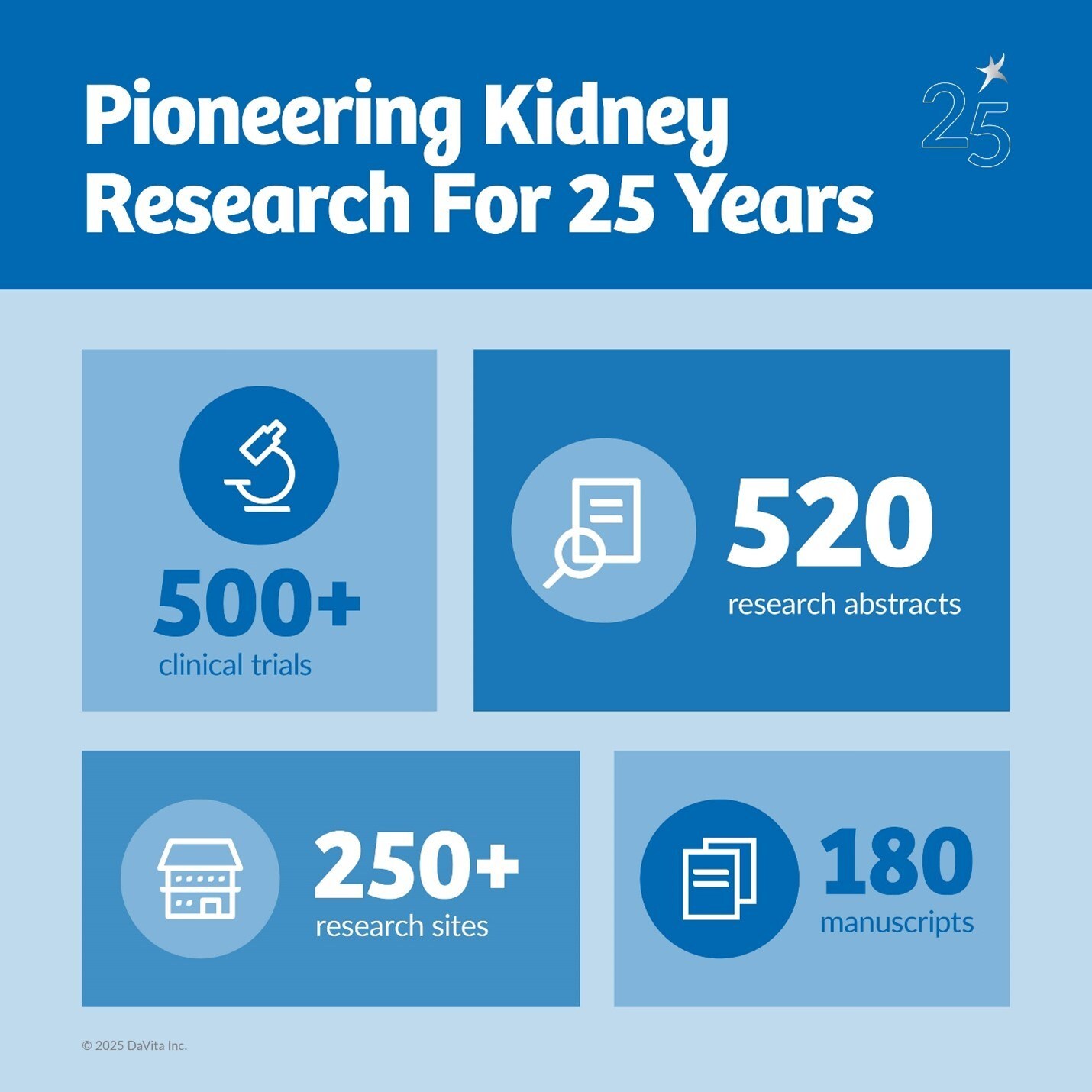
Research that Drives Results
DaVita has authored 180 manuscripts and 520 research abstracts or posters aimed at improving clinical outcomes in kidney care and adjacent disease states.
A 2017 study across 40 dialysis centers showed that ClearGuard HD antimicrobial barrier caps significantly reduce bloodstream infections for patients with central venous catheters (CVCs), improving outcomes at scale. CVCs can contribute disproportionately to bloodstream infection and, by extension, to infection-related hospitalization, mortality and morbidity in dialysis patients. The findings from this study yielded an important breakthrough, driving learnings across the nephrology community that helped improve patient outcomes at scale.
The most recent example is a forthcoming oral presentation to be presented at the American Society of Nephrology Kidney Week in November illustrating the association between GLP-1 drugs and hospitalization rates for people with kidney failure. This research originated from DaVita's program inviting independent medical directors to submit research ideas, ensuring studies reflect the real-world perspectives of prescribing nephrologists.
Clinical Trials that Advance Therapies
As the largest U.S. trial network for chronic kidney disease (CKD) and end stage kidney disease (ESKD), DCR leverages its broad reach and deep expertise to conduct impactful trials efficiently.
"By expanding our global research network, we're accelerating innovation and creating new opportunities to advance kidney care and related therapies," said Cristina Green, vice president of DCR.
Over the past 25 years, DCR has:
Expanding access through inclusive research
DaVita emphasizes outcomes research that addresses barriers faced by dialysis patients — an area often overlooked. In 2024, DCR implemented decentralized recruitment services to better include diverse patient populations in clinical trials. By leveraging DaVita's CKD electronic health record (EHR) system by Epic, this initiative has expanded trial access to underserved research locations.
For more information on DaVita' milestones over the last 25 years, visit DaVitaForward.com.
About DaVita
DaVita (NYSE: DVA) is a health care provider focused on transforming care delivery to improve quality of life for patients globally. As a comprehensive kidney care provider, DaVita has been a leader in clinical quality and innovation for 25 years. DaVita cares for patients at every stage and setting along their kidney health journey—from slowing the progression of kidney disease to helping to support transplantation, from acute hospital care to dialysis at home. As of June 30, 2025, DaVita served approximately 283,100 patients at 3,175 outpatient dialysis centers, of which 2,662 centers were located in the United States and 513 centers were located in 13 other countries worldwide. DaVita has reduced hospitalizations, improved mortality, helped improve health access and worked collaboratively to propel the kidney care community to adopt a higher quality standard of care for all patients, everywhere. To learn more, visit DaVita.com/About.
Media Contact
DaVita Newsroom
This email address is being protected from spambots. You need JavaScript enabled to view it.

| Last Trade: | US$121.35 |
| Daily Change: | 1.02 0.85 |
| Daily Volume: | 765,000 |
| Market Cap: | US$8.570B |
December 11, 2025 October 29, 2025 August 05, 2025 May 12, 2025 | |

Terns Pharmaceuticals is a clinical-stage biopharmaceutical company developing a portfolio of small-molecule product candidates to address serious diseases, including oncology and obesity. Terns’ pipeline contains three clinical stage development programs including GLP-1 receptor...
CLICK TO LEARN MORE
ClearPoint Neuro is a global therapy-enabling platform company providing stereotactic navigation and delivery to the brain. Applications of our ClearPoint Neuro Navigation System include electrode lead placement, placement of catheters, and biopsy. The platform has FDA clearance and is...
CLICK TO LEARN MOREEnd of content
No more pages to load