SAN DIEGO, May 10, 2023 (GLOBE NEWSWIRE) -- Belite Bio, Inc (NASDAQ: BLTE) (“Belite” or the “Company”), a clinical stage biopharmaceutical drug development company focused on advancing novel therapeutics targeting retinal degenerative eye diseases which have significant unmet medical needs, today announced its financial results for the first quarter ended March 31, 2023 and provided a general business update.
“The promising interim data after 18 months of treatment in our Phase 2 trial further reinforces the potential of Tinlarebant, an oral, once daily retinol binding protein antagonist, to slow disease progression in STGD1,” said Dr. Tom Lin, Belite’s Chairman and CEO. “We remain focused on advancing late-stage development of Tinlarebant and are pleased with the 60% enrollment seen to date in our pivotal Phase 3 DRAGON trial. We remain on track to enroll the first patient in our Phase 3 PHOENIX trial in GA in mid-2023.”
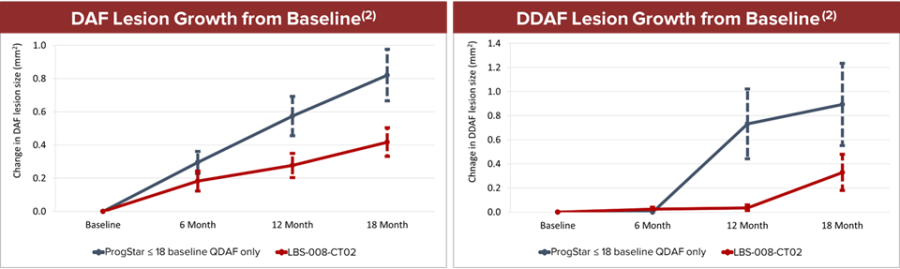
Dr. Nathan L. Mata, Chief Scientific Officer of Belite Bio added, “The 18-month data from our Phase 2 trial presented at ARVO are encouraging and highlight the ability of Tinlarebant to slow expansion of autofluorescence and the majority of the subjects showed no transition to atrophic (DDAF) lesions. Among the minority subjects who did transition to DDAF lesions, Tinlarebant slowed the rate of progression compared to those reported in a natural history study. We believe these 18-month Phase 2 data continue to reinforce the potential of Tinlarebant to be a transformative oral treatment for patients with STGD1.”
First Quarter 2023 Business Highlights and Upcoming Milestones:
Clinical Highlights
Tinlarebant (LBS-008) is designed to be an oral, potent, once daily retinol binding protein 4 (RBP4) antagonist that decreases RBP4 levels in the blood and selectively lowers vitamin A (retinol) delivery to the eye without disrupting systemic lipoprotein delivery to other tissues. Vitamin A is critical to normal vision but can accumulate as toxic byproducts and lead to retinal cell death and vision loss in certain eye diseases like STGD1 and GA, the advanced form of dry Age-Related Macular Degeneration (dry AMD).
Corporate Highlights
First Quarter 2023 Financial Results:
Cash and Cash Equivalents: As of March 31, 2023, the Company had $37.8 million in cash.
R&D Expenses:
For the three months ended March 31, 2023, research and development expenses were $5.7 million compared to $0.9 million for the same period in 2022. The increase in research and development expenses was primarily attributable to an increase in (i) research for and activities related to conducting DRAGON and PHOENIX trials, and (ii) wages and salaries due to our R&D team expansion.
G&A Expenses:
For the three months ended March 31, 2023, general and administration expenses were $1.2 million compared to $0.2 million for the same period in 2022. The increase in general and administration expenses was primarily due to increases in professional service fees, insurance premium for directors’ and officers’ liability insurance, and wages and salaries.
Net Loss:
For the three months ended March 31, 2023, the Company reported a net loss of $6.9 million, compared to a net loss of $1.1 million for the same period in 2022.
Webcast Information
Belite Bio will host a webcast to discuss the Company’s financial results and provide a business update. The call is scheduled for Thursday, May 11, 2023, at 8:00 a.m. Eastern Time. To join the live webcast please click here. A replay will be available approximately two hours after the event for 90 days.
About Belite Bio
Belite Bio is a clinical stage biopharmaceutical drug development company focused on advancing novel therapeutics targeting retinal degenerative eye diseases with significant unmet medical needs, such as STGD1 and GA in advanced dry AMD, in addition to specific metabolic diseases. For more information, follow us on Twitter, Instagram, LinkedIn, Facebook or visit us at www.belitebio.com.
Important Cautions Regarding Forward Looking Statements
This press release contains forward-looking statements about future expectations, plans and prospects, as well as any other statements regarding matters that are not historical facts. These statements include but are not limited to statements regarding the potential implications of clinical data for patients, clinical development, regulatory milestones, and commercialization of its product candidates, and any other statements containing the words “expect”, “will”, “believe”, “target”, and other similar expressions. Actual results may differ materially from those indicated in the forward-looking statements as a result of various important factors, including but not limited to Belite Bio’s ability to demonstrate the safety and efficacy of its drug candidates; the clinical results for its drug candidates, which may not support further development or regulatory approval; expectations for the timing of initiation, enrollment and completion of, and data relating to, its clinical trials; the content and timing of decisions made by the relevant regulatory authorities regarding regulatory approval of Belite Bio’s drug candidates; whether additional clinical trials may be required for DRAGON or PHOENIX studies based on their respective data; the potential efficacy of Tinlarebant, as well as those risks more fully discussed in the “Risk Factors” section in Belite Bio’s filings with the U.S. Securities and Exchange Commission. All forward-looking statements are based on information currently available to Belite Bio, and Belite Bio undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required by law.
| BELITE BIO, INC | |||||||||
| UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS | |||||||||
| (Amounts in thousands of US Dollars, except share and per share amounts) | |||||||||
| For the Three Months | |||||||||
| Ended March 31, | |||||||||
| 2022 | 2023 | ||||||||
| Expenses | |||||||||
| Research and development | 878 | 5,723 | |||||||
| General and administrative | 175 | 1,158 | |||||||
| Total operating expenses | 1,053 | 6,881 | |||||||
| Loss from operations | (1,053 | ) | (6,881 | ) | |||||
| Other income (expense): | |||||||||
| Total other (expense) income, net | (17 | ) | (8 | ) | |||||
| Loss before income tax | (1,070 | ) | (6,889 | ) | |||||
| Income tax expense | - | 6 | |||||||
| Net loss | (1,070 | ) | (6,895 | ) | |||||
| Other comprehensive income (loss) | |||||||||
| Foreign currency translation adjustments, net of nil tax | 25 | 16 | |||||||
| Total comprehensive loss | $ | (1,045 | ) | (6,879 | ) | ||||
| Weighted average number of ordinary shares used in per share calculation: | |||||||||
| - Basic and Diluted | 10,274,403 | 20,950,240 | |||||||
| Net loss per ordinary share | |||||||||
| - Basic and Diluted | $ | (0.10 | ) | $ | (0.33 | ) | |||
| BELITE BIO, INC | |||||||
| UNAUDITED CONDENSED CONSOLIDATED BALANCE SHEETS | |||||||
| (Amounts in thousands of US Dollars, except share amounts) | |||||||
| December 31, | March 31, | ||||||
| 2022 | 2023 | ||||||
| Current assets | $ | 42,807 | $ | 38,423 | |||
| Other assets | 1,466 | 1,395 | |||||
| TOTAL ASSETS | $ | 44,273 | $ | 39,818 | |||
| TOTAL LIABILITIES | $ | 2,772 | $ | 4,649 | |||
| TOTAL SHAREHOLDERS’ EQUITY | 41,501 | 35,169 | |||||
| TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY | $ | 44,273 | $ | 39,818 | |||
| Ordinary shares authorized | 492,179,086 | 400,000,000 | |||||
| Ordinary shares issued and outstanding | 24,898,908 | 24,914,741 | |||||
Media and Investor Relations Contact:
Jennifer Wu /This email address is being protected from spambots. You need JavaScript enabled to view it.
Tim McCarthy /This email address is being protected from spambots. You need JavaScript enabled to view it.
| Note: | (1) Although we have compared the published data for the above studies to our interim data in the LBS-008-CT02 trial, the value of such comparisons is limited because they are derived from studies conducted under different protocols, at different sites, with different patient populations, and results were analyzed using non-standardized methods. We note our future trials may not confirm the comparisons or analyses we have made to date. |
| (2) For each subject, the mean lesion size between eyes was determined and the average value for the lesion sizes within each study group was calculated. The data show mean lesion sizes within each group ± standard error of the mean at each timepoint. | |

| Last Trade: | US$136.00 |
| Daily Change: | 6.38 4.92 |
| Daily Volume: | 73,746 |
| Market Cap: | US$4.750B |
November 10, 2025 September 12, 2025 September 08, 2025 | |

C4 Therapeutics is pioneering a new class of small-molecule drugs that selectively destroy disease-causing proteins via degradation using the innate machinery of the cell. This targeted protein degradation approach offers advantages over traditional drugs, including the potential to treat a wider range of diseases...
CLICK TO LEARN MORE
Immix Biopharma is a clinical-stage biopharmaceutical company pioneering a novel class of CAR-T cell therapies and Tissue-Specific Therapeutics targeting oncology and immuno-dysregulated diseases with >75 patients treated to-date. Our lead cell therapy asset is NXC-201...
CLICK TO LEARN MOREEnd of content
No more pages to load