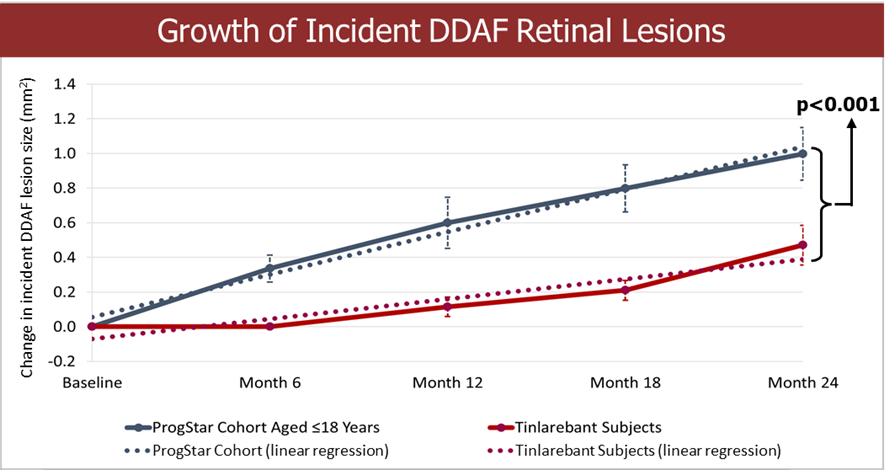
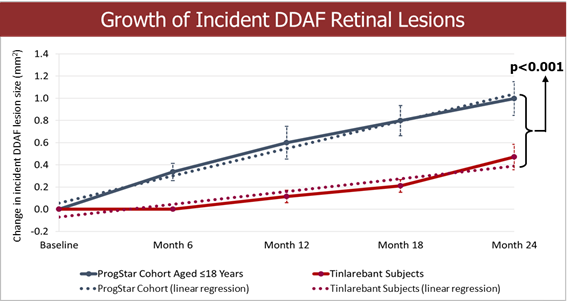
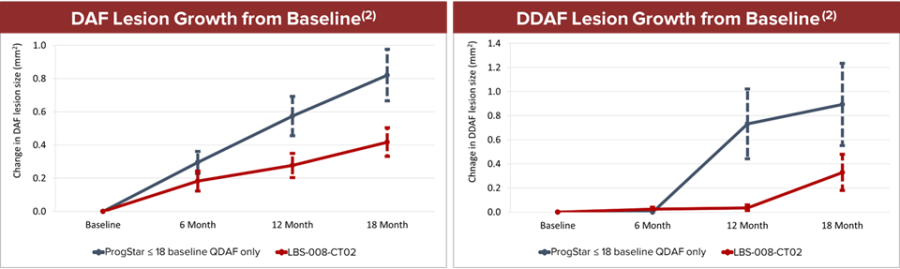
Completed enrollment with 530 subjects in the pivotal phase 3 PHOENIX trial in geographic atrophy (GA) Completed $15 million registered direct offering and $125 million private placement with potential for up to an additional $165 million upon full warrant exercise Completed pivotal phase 3 DRAGON trial in Stargardt disease (STGD); final topline data expected in Q4 2025 China’s NMPA has agreed to accept New Drug Application... Read More