DALLAS, March 19, 2024 (GLOBE NEWSWIRE) -- Taysha Gene Therapies, Inc. (Nasdaq: TSHA) (Taysha or the Company), a clinical-stage gene therapy company focused on developing and commercializing AAV-based gene therapies for the treatment of severe monogenic diseases of the central nervous system (CNS), today reported financial results for the full-year ended December 31, 2023, and provided corporate and clinical updates.
“We are highly encouraged by the safety profile and durable response reported in the longer-term data from the low dose cohort in our REVEAL adolescent and adult trial. Importantly, following completion of the steroid taper for the first patient and at decreased steroid levels for the second patient, both patients showed sustained improvements across multiple clinical domains, as well as new improvements compared to earlier post-treatment assessments, which supports the transformative potential of TSHA-102,” said Sean P. Nolan, Chairman and Chief Executive Officer of Taysha. “These continued improvements in both adult patients with advanced stage four Rett syndrome and the initial clinical data from the first pediatric patient were reviewed by the Independent Data Monitoring Committee (IDMC) and enabled us to proceed to earlier dose escalation in the adolescent and adult trial, which will expedite and further inform our clinical development and regulatory strategy for the dose expansion portion of the studies. With this progress, we believe we are well-positioned to focus on generating clinical data in a broad range of ages and stages of patients with Rett syndrome across multiple geographies this year.”
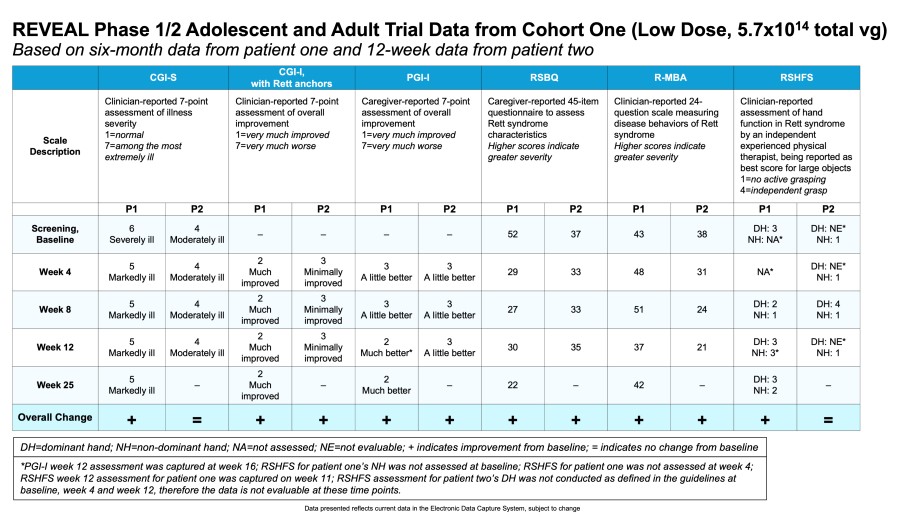
Dr. Elsa Rossignol, M.D., FRCP, FAAP, Associate Professor in Neuroscience and Pediatrics at the Université de Montréal, and Principal Investigator of the REVEAL trial at the CHU Sainte-Justine added, “Both adult patients treated with TSHA-102 showed sustained and new improvements across key areas of disease impacting activities of daily living, including multiple aspects of autonomic function, social communication, motor skills, and experienced stabilized or significantly reduced seizures. The first patient sustained improvements at week 35 post-treatment after the completion of her steroid taper, with restored movement in her legs, the gained ability to sit unassisted for the first time in over a decade and gained function in her non-dominant hand. She has also sustained improvements in breathing dysrhythmia and sleep quality and duration, including the gained ability to sleep through the night for the first time in 20 years. Notably, she has vastly increased interest in social communication and activities at week 35 compared to earlier post-treatment assessments. She is more alert and socially interactive, with increased vocalizations and enhanced ability to use an eye-driven communication device. The second patient showed sustained improvements at decreased steroid levels at week 19 post-treatment, including reduced hand stereotypies for the first time since regression at age three, and sustained improvements in breathing dysrhythmia, including hyperventilation and reduced apneic spells. As of week 19 post-treatment, she’s experienced a significant reduction in seizures at a lower dose of anti-seizure medication. Collectively, these continued improvements in both adult patients with different genetic mutation severity and phenotypic expression are encouraging and support the potential of TSHA-102 to bring meaningful change to the lives of patients and their caregivers.”
Data Summary from Cohort One (Low Dose, 5.7x1014 total vg) of the REVEAL Phase 1/2 Adolescent and Adult Trial
TSHA-102 in Rett syndrome: a self-complementary intrathecally delivered AAV9 gene transfer therapy in clinical evaluation for Rett syndrome, a rare genetic neurodevelopmental disorder caused by mutations in the X-linked MECP2 gene. TSHA-102 utilizes a novel miRARE technology designed to mediate levels of MECP2 in the CNS on a cell-by-cell basis without risk of overexpression. The safety and preliminary efficacy of TSHA-102 are being evaluated in female patients aged 12-years and older with Rett syndrome due to MECP2 loss-of-function mutation in the REVEAL Phase 1/2 adolescent and adult trial, a first-in-human, open-label, randomized, dose-escalation and dose-expansion study taking place in Canada and the United States (U.S.). Dose escalation will evaluate two dose levels of TSHA-102 sequentially. The maximum tolerated dose (MTD) or maximum administered dose (MAD) established in Part A will then be administered during dose expansion in Part B of the study.
Results from the first patient (large MECP2 deletion; associated with severe phenotype) and second patient (missense MECP2 mutation; associated with milder phenotype) with late motor deterioration stage four Rett syndrome dosed with TSHA-102 in the low dose cohort (5.7x1014 total vg):
Recent Corporate and Program Highlights
Anticipated 2024 Milestones
Full-Year 2023 Financial Highlights
Revenue: Revenue for the full year ended December 31, 2023, was $15.5 million compared to $2.5 million for the full year ended December 31, 2022, as revenue was derived entirely from the Company’s Option Agreement with Audentes Therapeutics, Inc. (d/b/a Astellas Gene Therapies). The increase in revenue is primarily a result of Rett syndrome research and development activities performed in 2023.
Research and Development Expenses: Research and development expenses were $56.8 million for the full year ended December 31, 2023, compared to $91.2 million for the full year ended December 31, 2022. The decrease was due to reduced research and development headcount, lower research and development manufacturing expenses and a reduction in third-party research and development consulting fees, mainly related to pre-clinical studies and IND-enabling toxicology studies.
General and Administrative Expenses: General and administrative expenses were $30.0 million for the full year ended December 31, 2023, compared to $37.4 million for the full year ended December 31, 2022. The decrease was primarily attributable to a reduction in compensation expenses as a result of lower headcount and reduced corporate insurance and consulting expenses.
Net loss: Net loss for the full year ended December 31, 2023, was $111.6 million, or $0.96 per share, as compared to a net loss of $166.0 million, or $3.78 per share, for the full year ended December 31, 2022. The net loss includes a non-recurring and non-cash expense of $34.5 million related to the change in fair value from the pre-funded warrants as a result of the August 2023 private placement financing.
Cash and cash equivalents: As of December 31, 2023, Taysha had $143.9 million in cash and cash equivalents. The Company continues to expect that its current cash resources will support planned operating expenses and capital requirements into 2026.
Conference Call and Webcast Information
Taysha management will hold a conference call and webcast today at 4:30 p.m. ET to review its financial and operating results and to provide corporate and clinical updates. The dial-in number for the conference call is 877-407-0792 (U.S./Canada) or 201-689-8263 (international). The conference ID for all callers is 13744574. The live webcast and replay may be accessed by visiting Taysha’s website at https://ir.tayshagtx.com/news-events/events-presentations. An archived version of the webcast will be available on the website for 30 days.
About TSHA-102
TSHA-102 is a self-complementary intrathecally delivered AAV9 investigational gene transfer therapy in clinical evaluation for Rett syndrome. Designed as a one-time treatment, TSHA-102 aims to address the genetic root cause of the disease by delivering a functional form of MECP2 to cells in the CNS. TSHA-102 utilizes a novel miRNA-Responsive Auto-Regulatory Element (miRARE) technology designed to mediate levels of MECP2 in the CNS on a cell-by-cell basis without risk of overexpression. TSHA-102 has received Fast Track designation and Orphan Drug and Rare Pediatric Disease designations from the FDA and has been granted Orphan Drug designation from the European Commission. TSHA-102 has also received Innovative Licensing and Access Pathway designation from the U.K. Medicines and Healthcare Products Regulatory Agency.
About Rett Syndrome
Rett syndrome is a rare neurodevelopmental disorder caused by mutations in the X-linked MECP2 gene encoding methyl CpG-binding protein 2 (MeCP2), which is essential for regulating neuronal and synaptic function in the brain. The disorder is characterized by loss of communication and hand function, slowing and/or regression of development, motor and respiratory impairment, seizures, intellectual disabilities and shortened life expectancy. Rett syndrome progression is divided into four key stages, beginning with early onset stagnation at 6 to 18 months of age followed by rapid regression, plateau and late motor deterioration. Rett syndrome primarily occurs in females and is one of the most common genetic causes of severe intellectual disability. Currently, there are no approved disease-modifying therapies that treat the genetic root cause of the disease. Rett syndrome caused by a pathogenic/likely pathogenic MECP2 mutation is estimated to affect between 15,000 and 20,000 patients in the U.S., EU, and U.K.
About Taysha Gene Therapies
Taysha Gene Therapies (Nasdaq: TSHA) is a clinical-stage biotechnology company focused on advancing adeno-associated virus (AAV)-based gene therapies for severe monogenic diseases of the central nervous system. Its lead clinical program TSHA-102 is in development for Rett syndrome, a rare neurodevelopmental disorder with no approved disease-modifying therapies that address the genetic root cause of the disease. With a singular focus on developing transformative medicines, Taysha aims to address severe unmet medical needs and dramatically improve the lives of patients and their caregivers. The Company’s management team has proven experience in gene therapy development and commercialization. Taysha leverages this experience, its manufacturing process and a clinically and commercially proven AAV9 capsid in an effort to rapidly translate treatments from bench to bedside. For more information, please visit www.tayshagtx.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as “anticipates,” “believes,” “expects,” “intends,” “projects,” “plans,” and “future” or similar expressions are intended to identify forward-looking statements. Forward-looking statements include statements concerning the potential of TSHA-102, including the reproducibility and durability of any favorable results initially seen in patient dosed to date in clinical trials, and our other product candidates, to positively impact quality of life and alter the course of disease in the patients we seek to treat, our research, development and regulatory plans for our product candidates, including the timing of initiating additional trials and reporting data from our clinical trials, the potential for these product candidates to receive regulatory approval from the FDA or equivalent foreign regulatory agencies, and our current cash resources supporting our planned operating expenses and capital requirements into 2026. Forward-looking statements are based on management’s current expectations and are subject to various risks and uncertainties that could cause actual results to differ materially and adversely from those expressed or implied by such forward-looking statements. Accordingly, these forward-looking statements do not constitute guarantees of future performance, and you are cautioned not to place undue reliance on these forward-looking statements. Risks regarding our business are described in detail in our Securities and Exchange Commission (“SEC”) filings, including in our Annual Report on Form 10-K for the full-year ended December 31, 2023, which is available on the SEC’s website at www.sec.gov. Additional information will be made available in other filings that we make from time to time with the SEC. These forward-looking statements speak only as of the date hereof, and we disclaim any obligation to update these statements except as may be required by law.
| Taysha Gene Therapies, Inc. Condensed Consolidated Statements of Operations (in thousands, except share and per share data) | |||||||
| For the Year Ended December 31, | |||||||
| 2023 | 2022 | ||||||
| Revenue | $ | 15,451 | $ | 2,502 | |||
| Operating expenses: | |||||||
| Research and development | 56,778 | 91,169 | |||||
| General and administrative | 30,047 | 37,360 | |||||
| Impairment of long-lived assets | 1,065 | 36,420 | |||||
| Total operating expenses | 87,890 | 164,949 | |||||
| Loss from operations | (72,439 | ) | (162,447 | ) | |||
| Other income (expense): | |||||||
| Change in fair value of warrant liability | (34,718 | ) | — | ||||
| Loss on debt extinguishment | (1,398 | ) | — | ||||
| Change in fair value of term loan | (1,538 | ) | — | ||||
| Interest income | 3,572 | 249 | |||||
| Interest expense | (4,998 | ) | (3,798 | ) | |||
| Other expense | (47 | ) | (18 | ) | |||
| Total other expense, net | (39,127 | ) | (3,567 | ) | |||
| Net loss | $ | (111,566 | ) | $ | (166,014 | ) | |
| Net loss per common share, basic and diluted | $ | (0.96 | ) | $ | (3.78 | ) | |
| Weighted average common shares outstanding, basic and diluted | 116,121,482 | 43,952,015 | |||||
| Taysha Gene Therapies, Inc. Condensed Consolidated Balance Sheet Data (in thousands, except share and per share data) | |||||||
| December 31, 2023 | December 31, 2022 | ||||||
| ASSETS | |||||||
| Current assets: | |||||||
| Cash and cash equivalents | $ | 143,940 | $ | 87,880 | |||
| Restricted cash | 449 | — | |||||
| Prepaid expenses and other current assets | 3,479 | 8,537 | |||||
| Assets held for sale | 2,000 | — | |||||
| Total current assets | 149,868 | 96,417 | |||||
| Restricted cash | 2,151 | 2,637 | |||||
| Property, plant and equipment, net | 10,826 | 14,963 | |||||
| Operating lease right-of-use assets | 9,582 | 10,943 | |||||
| Other non-current assets | 304 | 1,316 | |||||
| Total assets | $ | 172,731 | $ | 126,276 | |||
| LIABILITIES AND STOCKHOLDERS' EQUITY | |||||||
| Current liabilities: | |||||||
| Accounts payable | $ | 6,366 | $ | 10,946 | |||
| Accrued expenses and other current liabilities | 12,284 | 18,287 | |||||
| Deferred revenue | 18,106 | 33,557 | |||||
| Total current liabilities | 36,756 | 62,790 | |||||
| Term loan, net | 40,508 | 37,967 | |||||
| Operating lease liability, net of current portion | 18,953 | 20,440 | |||||
| Other non-current liabilities | 1,577 | 4,130 | |||||
| Total liabilities | 97,794 | 125,327 | |||||
| Stockholders' equity | |||||||
| Preferred stock, $0.00001 par value per share; 10,000,000 shares authorized and no shares issued and outstanding as of December 31, 2023 and December 31, 2022 | — | — | |||||
| Common stock, $0.00001 par value per share; 400,000,000 shares authorized and 186,960,193 issued and outstanding as of December 31, 2023, and 200,000,000 shares authorized and 63,207,507 issued and outstanding as of December 31, 2022 | 2 | 1 | |||||
| Additional paid-in capital | 587,942 | 402,389 | |||||
| Accumulated deficit | (513,007 | ) | (401,441 | ) | |||
| Total stockholders’ equity | 74,937 | 949 | |||||
| Total liabilities and stockholders' equity | $ | 172,731 | $ | 126,276 | |||
Company Contact:
Hayleigh Collins
Director, Head of Corporate Communications and Investor Relations
Taysha Gene Therapies, Inc.
This email address is being protected from spambots. You need JavaScript enabled to view it.
Media Contact:
Carolyn Hawley
Inizio Evoke
This email address is being protected from spambots. You need JavaScript enabled to view it.

| Last Trade: | US$4.49 |
| Daily Change: | 0.43 10.59 |
| Daily Volume: | 3,137,582 |
| Market Cap: | US$1.230B |
November 04, 2025 October 16, 2025 October 02, 2025 August 12, 2025 | |

Chimerix is on a mission to develop medicines that meaningfully improve and extend the lives of patients facing deadly diseases. The company is devoted to filling gaps in the treatment paradigm. Chimerix’s most advanced clinical-stage program is in development for H3 K27M-mutant glioma....
CLICK TO LEARN MORE
Terns Pharmaceuticals is a clinical-stage biopharmaceutical company developing a portfolio of small-molecule product candidates to address serious diseases, including oncology and obesity. Terns’ pipeline contains three clinical stage development programs including GLP-1 receptor...
CLICK TO LEARN MOREEnd of content
No more pages to load