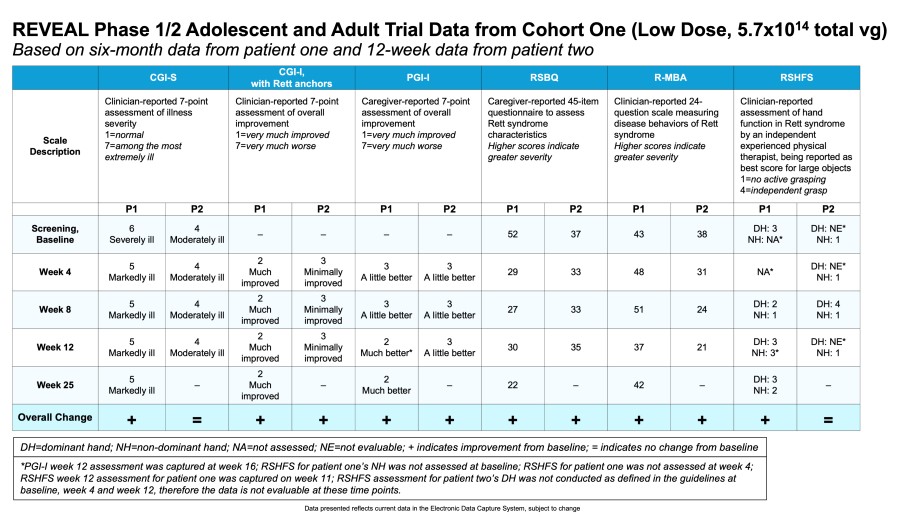
TSHA-102 granted Breakthrough Therapy designation by FDA Finalized FDA alignment on REVEAL pivotal trial protocol and SAP, including 6-month interim analysis that may expedite BLA submission, which was enabled by the rigorous developmental milestone evaluation in Part A REVEAL Phase 1/2 trials showing an unprecedented response rate Dosing of first patient in REVEAL pivotal trial scheduled for Q4 2025, with enrollment of... Read More