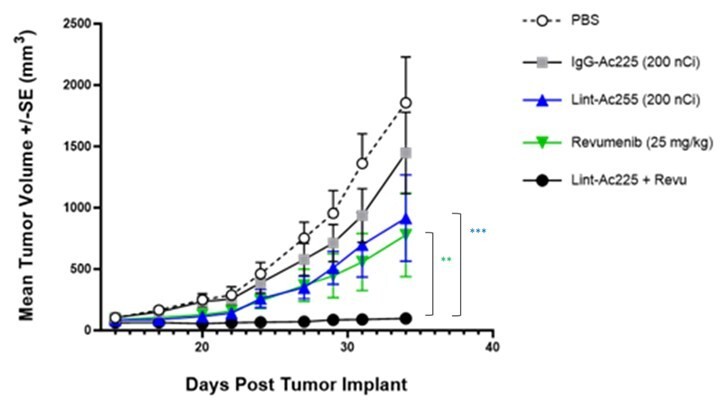
New preclinical data to show anti-tumor activity of ATNM-400 in hormone-resistant and HER2-resistant breast cancer, addressing the need for new treatment options beyond tamoxifen and trastuzumab ATNM-400 demonstrates efficacy across prostate cancer, non-small cell lung cancer (NSCLC), and breast cancer - three of the largest oncology indications - with potential as monotherapy, in combination, or as an alternative therapy... Read More