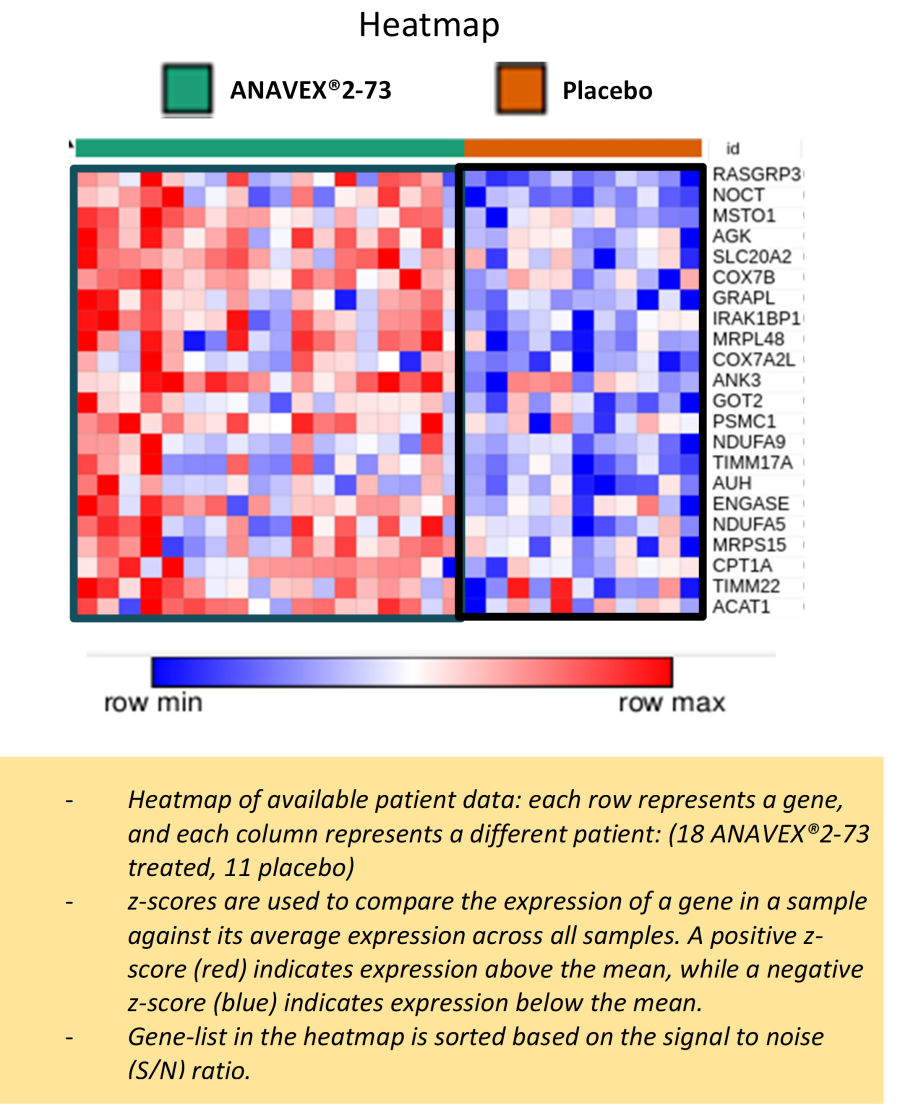
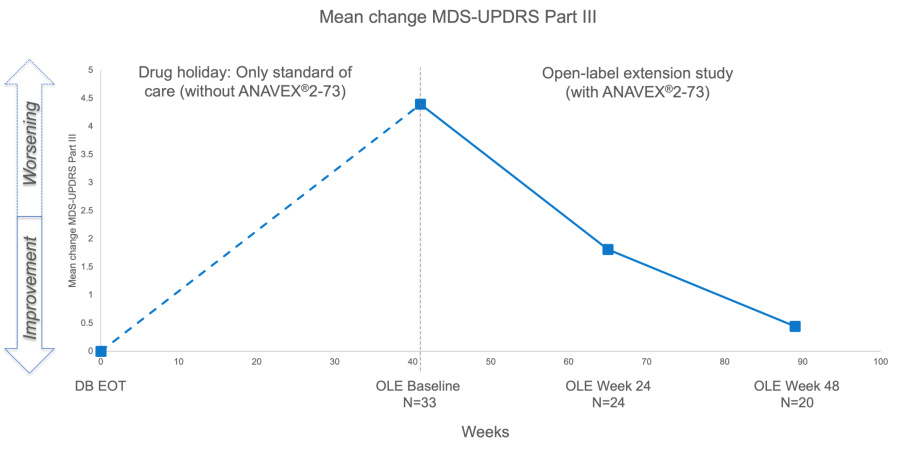
NEW YORK, Nov. 19, 2025 (GLOBE NEWSWIRE) -- Anavex Life Sciences Corp . (“Anavex” or the “Company”) (Nasdaq: AVXL), a clinical-stage biopharmaceutical company focused on developing innovative treatments for Alzheimer's disease, Parkinson's disease, schizophrenia, neurodevelopmental, neurodegenerative, and rare diseases, including Rett syndrome, and other central nervous system (CNS) disorders, today announced that it will... Read More