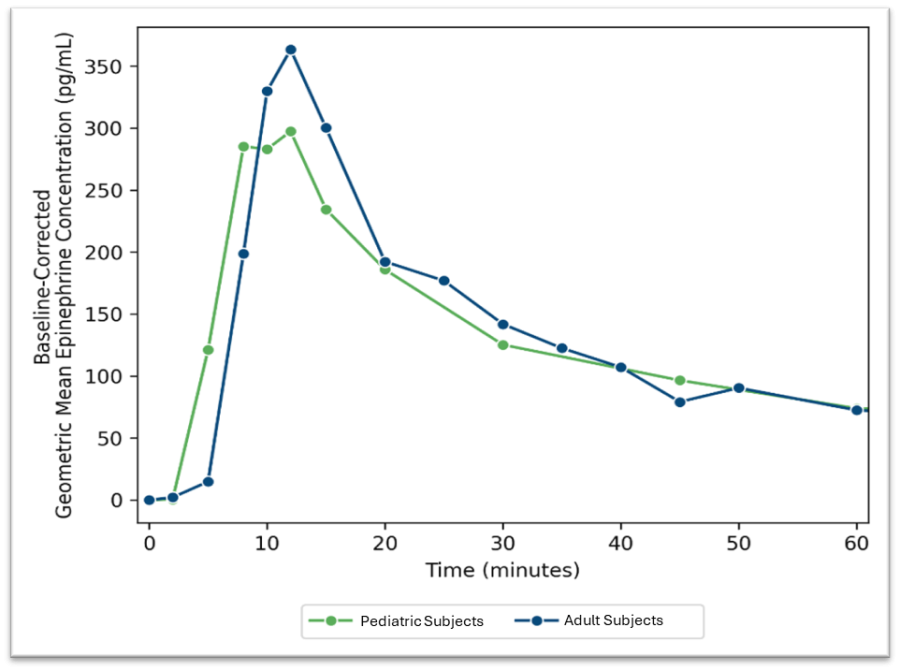
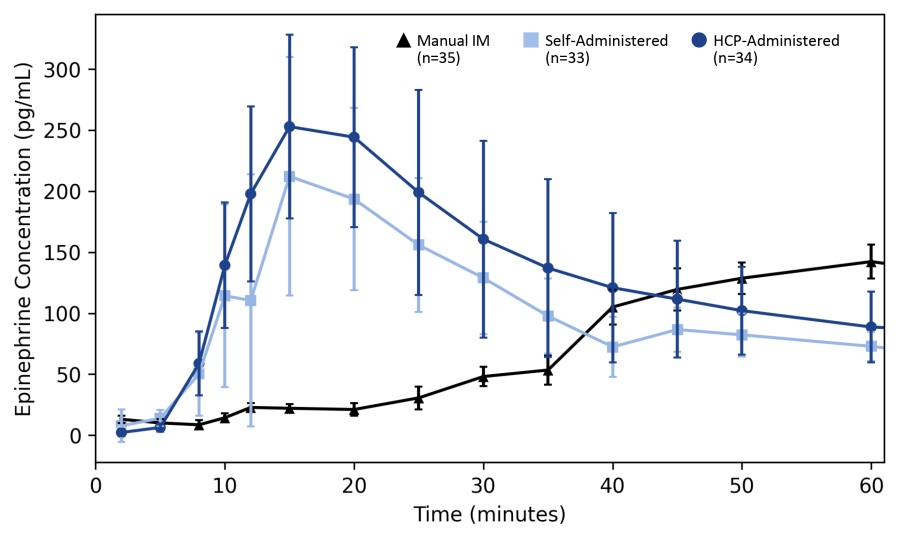
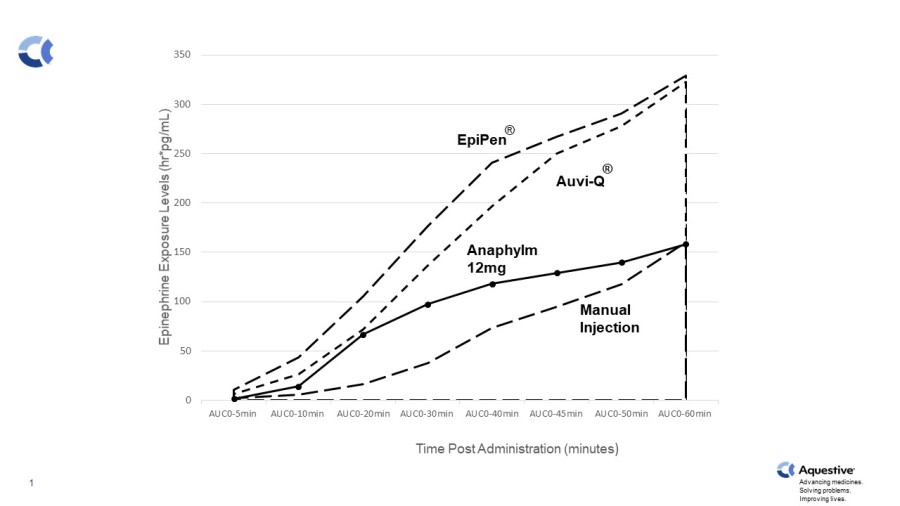
WARREN, N.J., Nov. 24, 2025 (GLOBE NEWSWIRE) -- Aquestive Therapeutics, Inc. (NASDAQ:AQST) ("Aquestive" or the "Company"), a pharmaceutical company advancing medicines to bring meaningful improvement to patients' lives through innovative science and delivery technologies, announced today that the Aquestive management team will participate in the upcoming Piper Sandler 37th Annual Healthcare Conference December 2-4, 2025, in... Read More