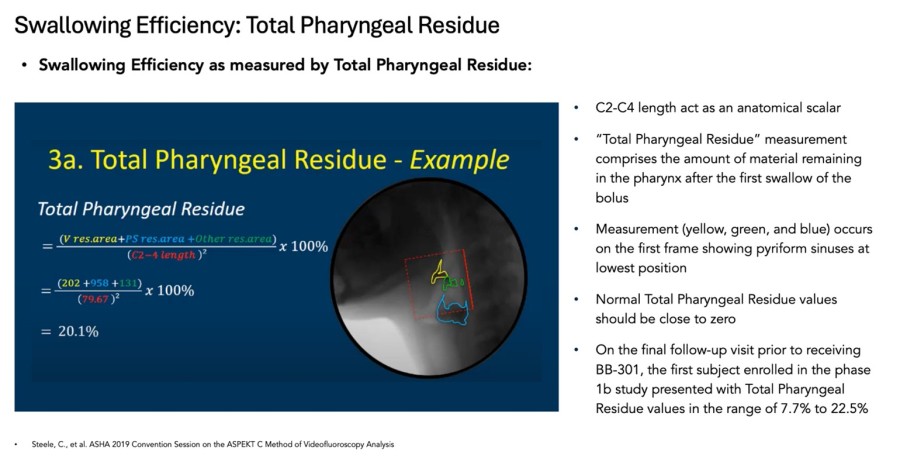
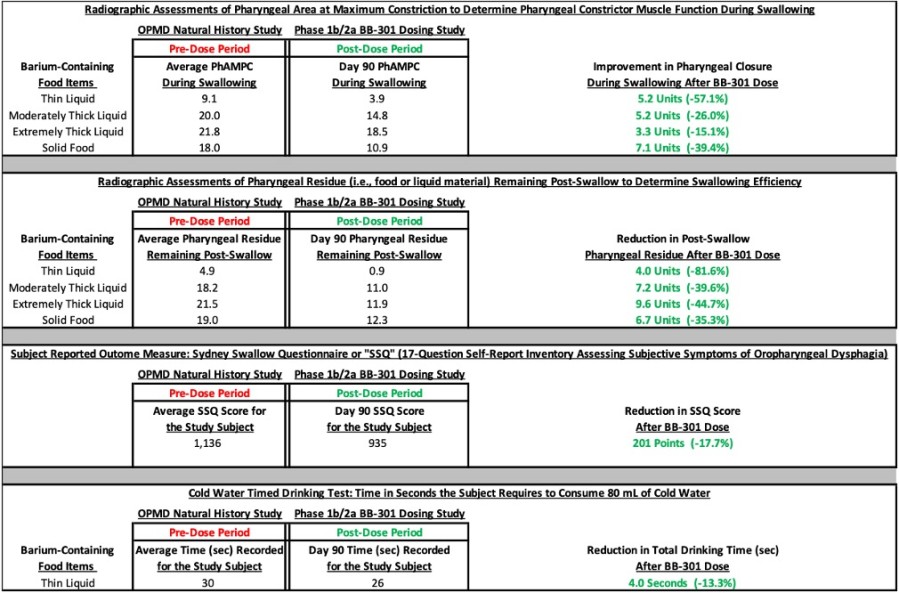
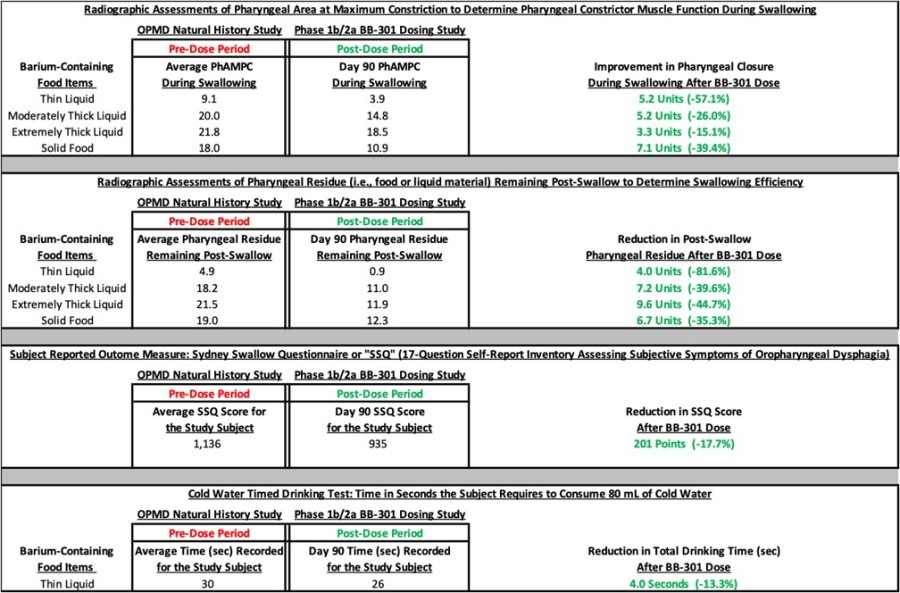
fast Track Designation was granted for BB-301 following U.S. Food and Drug Administration (FDA) review of positive interim clinical study results and proprietary Responder Analysis planned for use in pivotal study for BB-301 Positive interim clinical study results from the Phase 1b/2a trial of BB-301, showed a 100% responder rate, with all six patients in Cohort 1 meeting the formal statistical criteria for response to... Read More