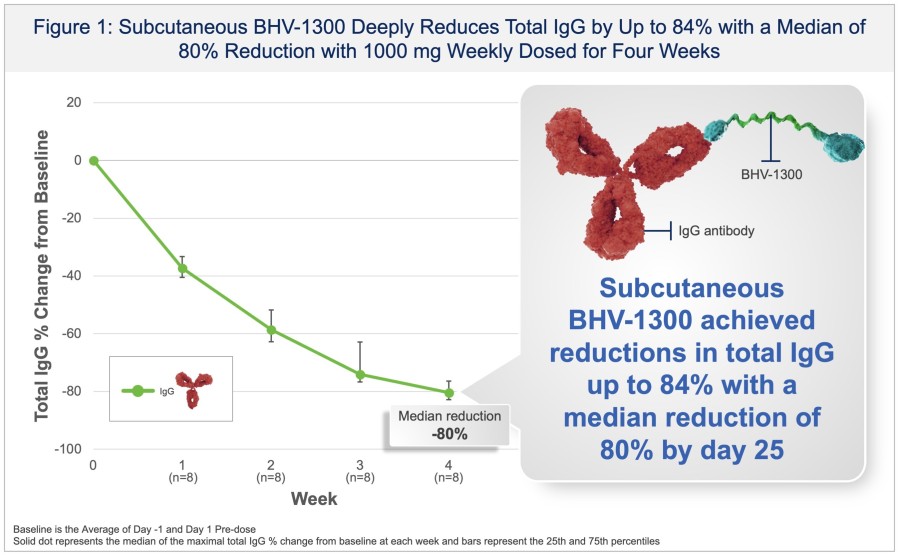
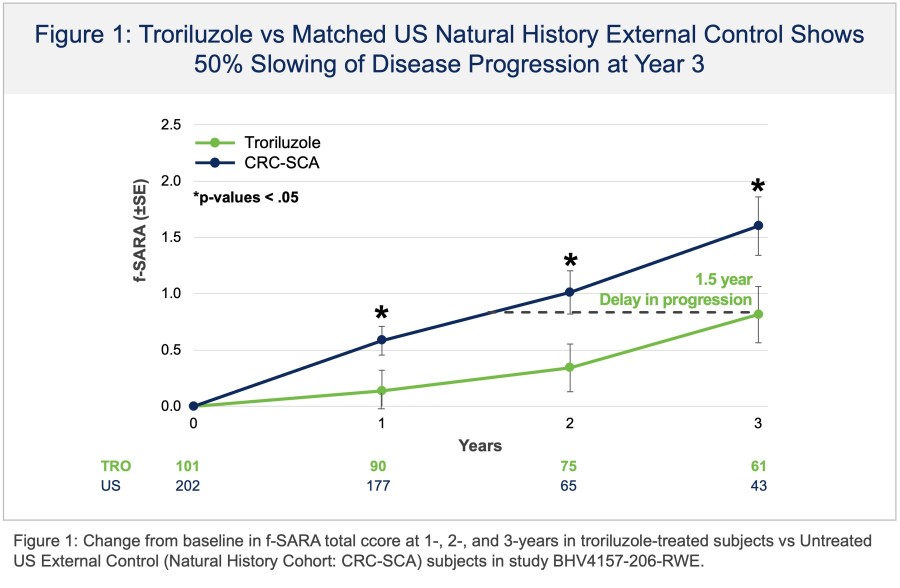
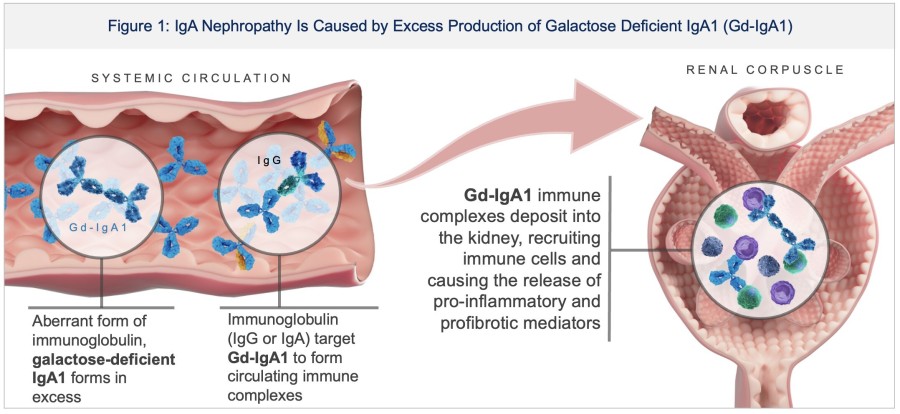
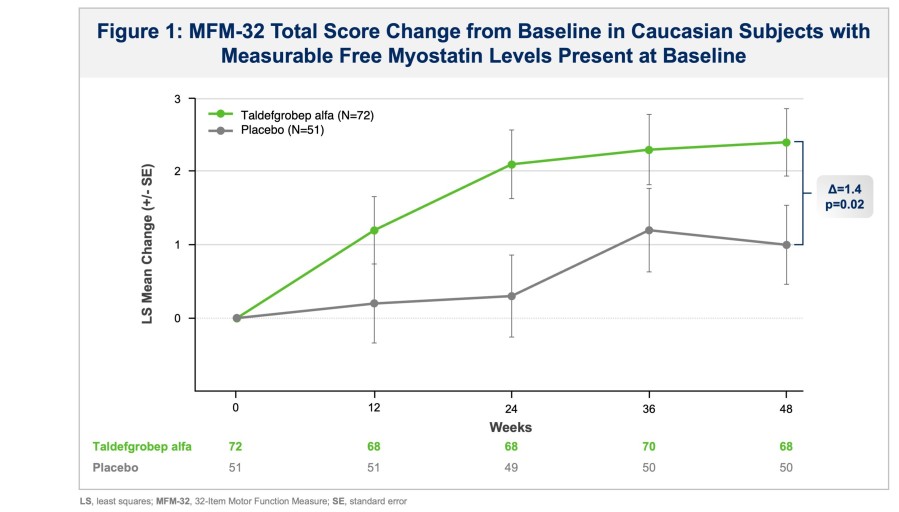
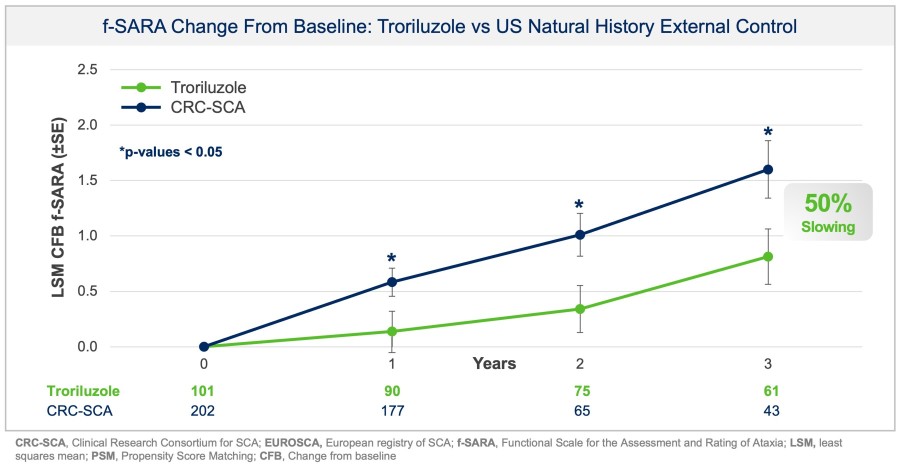
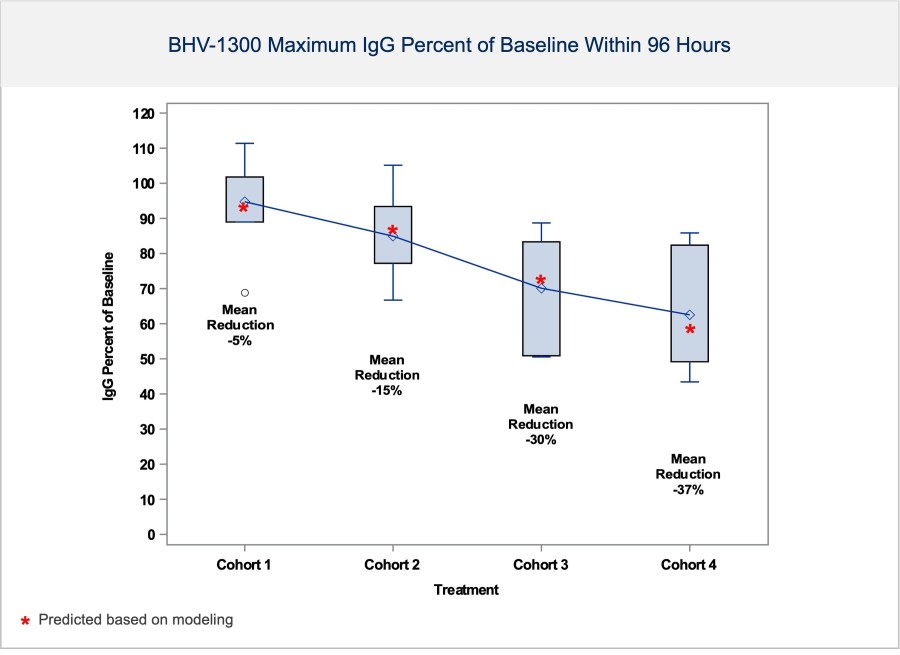
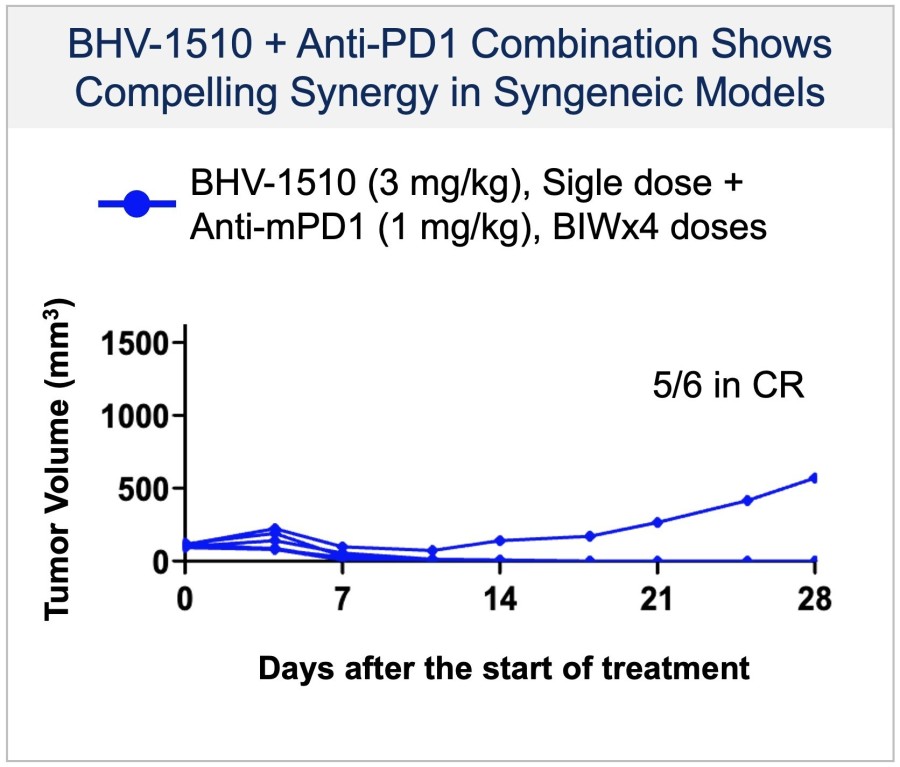
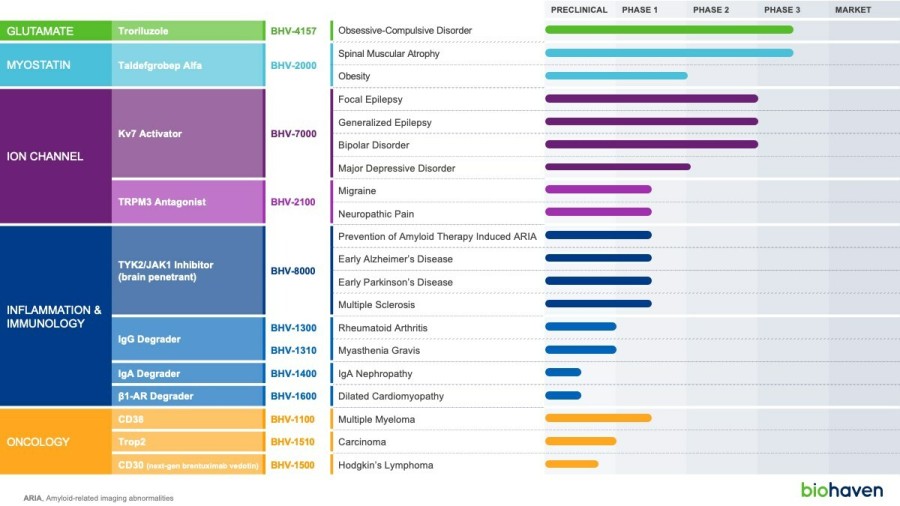
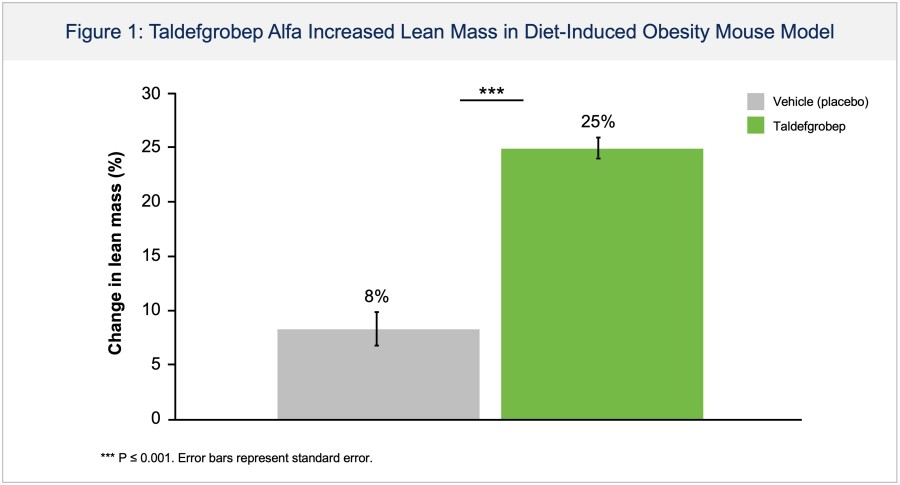
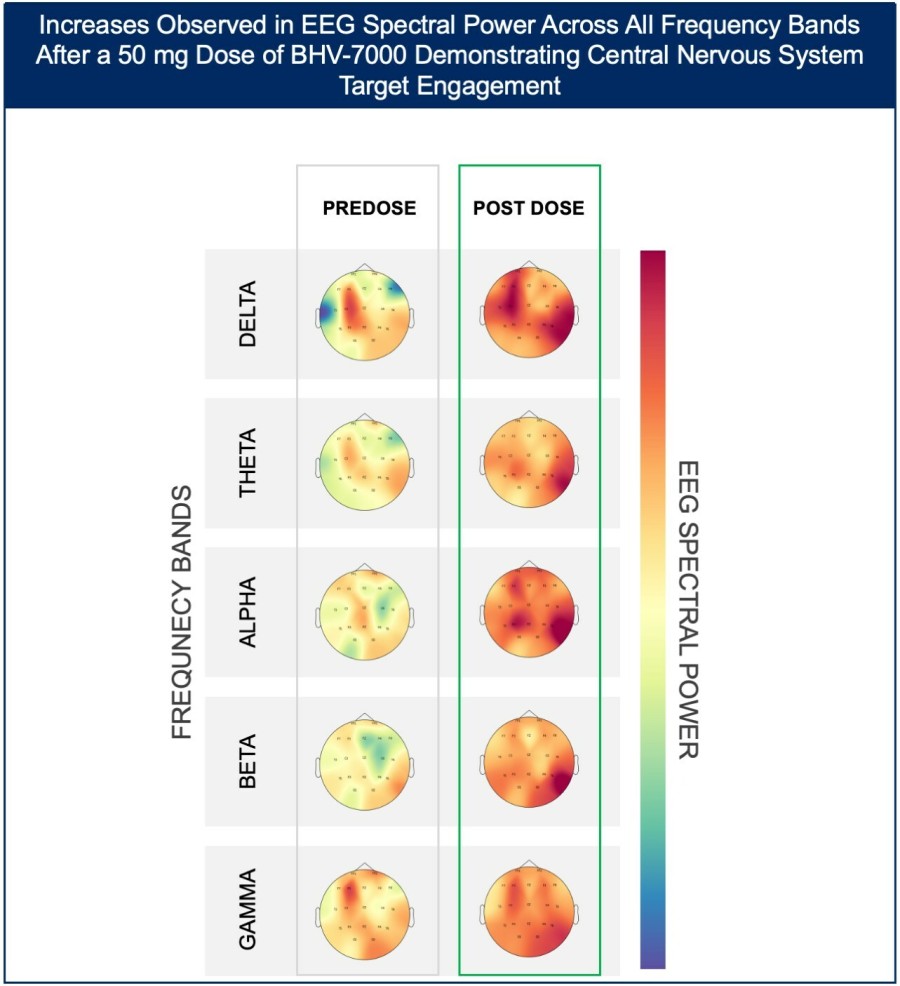
In a pretreated population of participants with advanced/metastatic cancer and the majority with prior PD-(L)1 treatment, BHV-1510 2.5 mg/kg Q3W plus cemiplimab resulted in confirmed objective response rates 3/5 (60%) in NSCLC, 4/4 (100%) in endometrial cancer, and 1/2 (50%) in urothelial cancer There were low rates of adverse events attributed to unconjugated payload such as hematological toxicities and diarrhea, and there... Read More