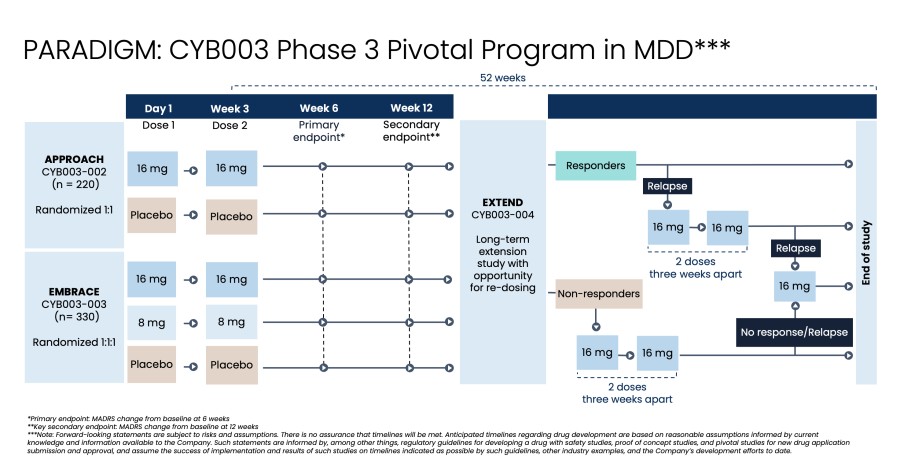
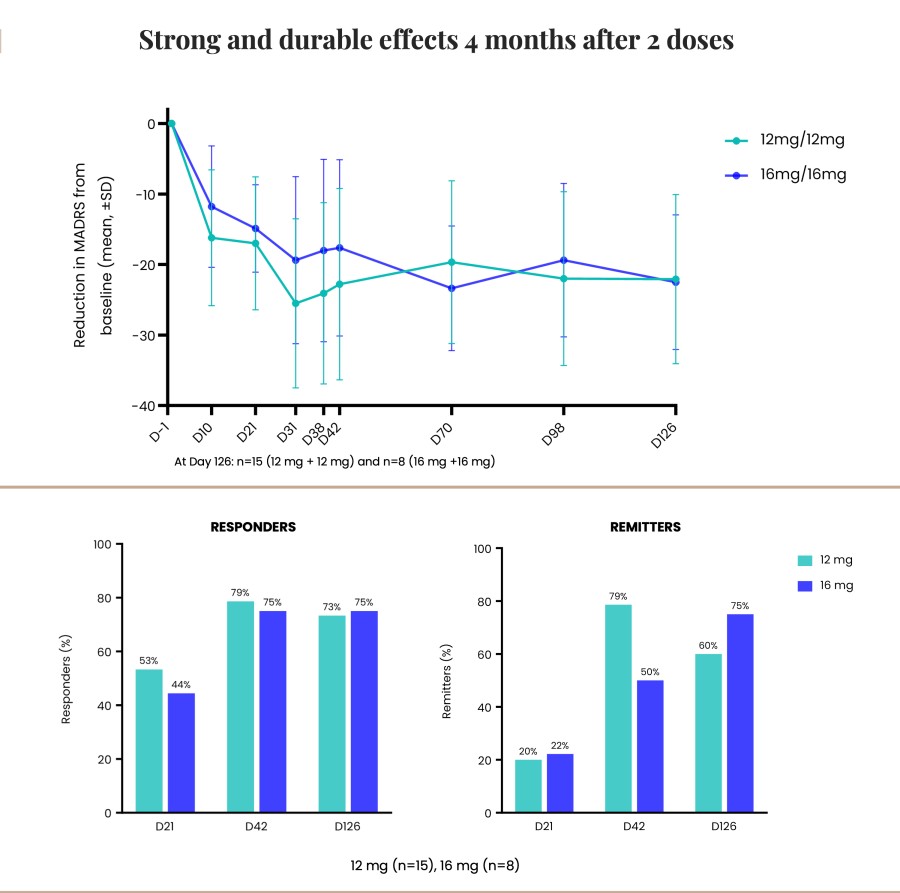
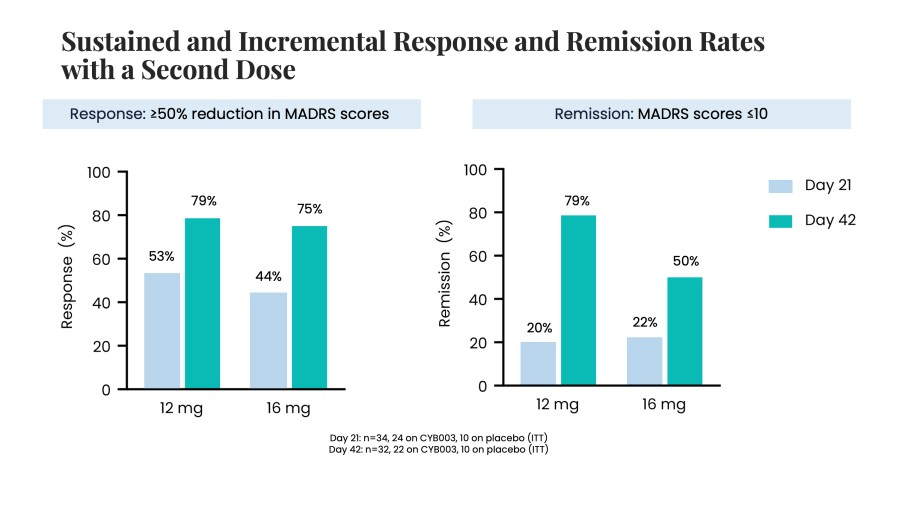
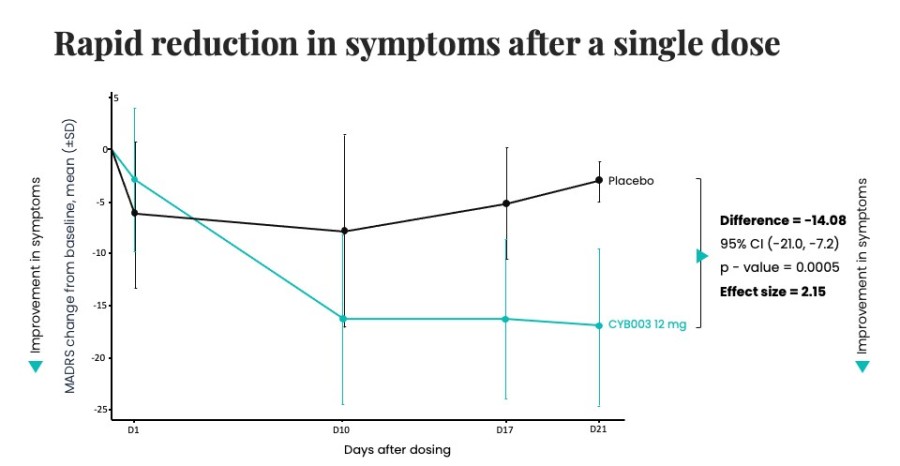
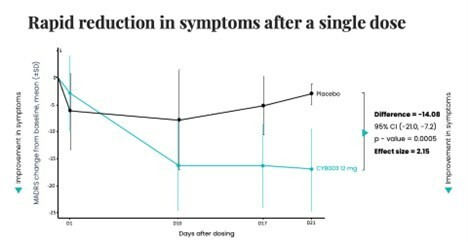
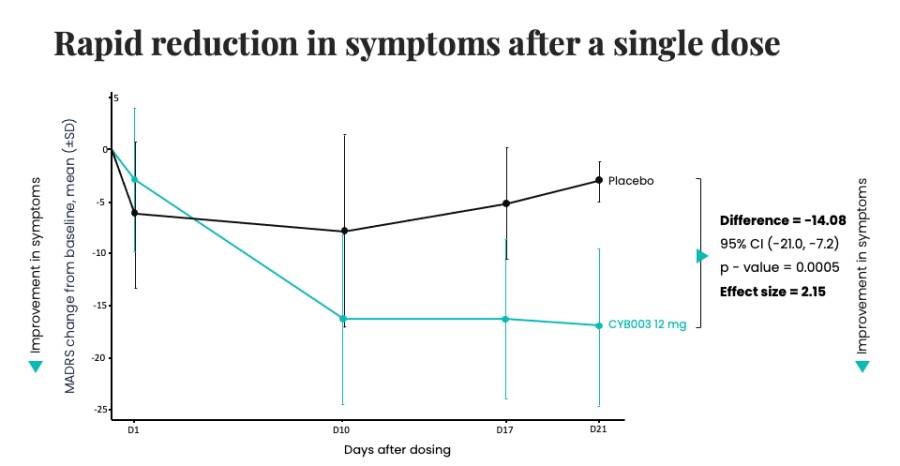
Closed registered direct offering for aggregate gross proceeds of US$175 million (the “Registered Direct Offering”) led by a syndicate of prominent biopharmaceutical institutional investors On track to report topline data from Phase 2 study evaluating CYB004 for the treatment of Generalized Anxiety Disorder (“GAD”) in Q1 2026 1 Continues to advance Phase 3 studies of CYB003 for the adjunctive treatment of major depressive... Read More