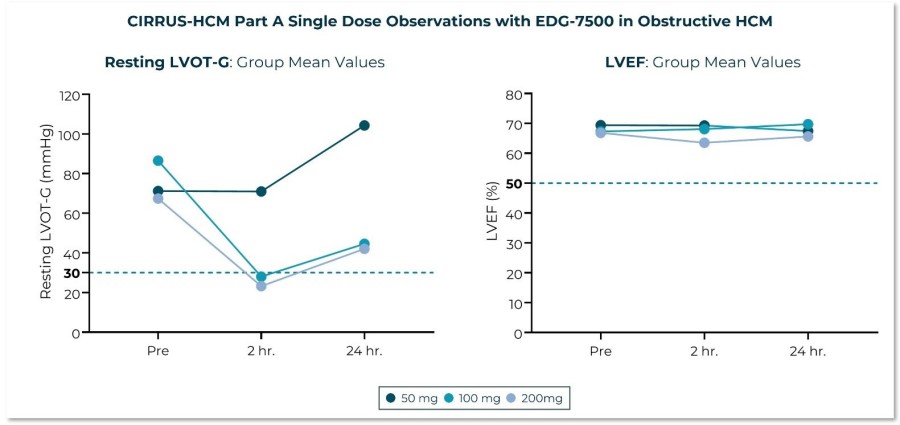
In the CIRRUS-HCM trial, including interim safety results from Part D, EDG-7500 was generally well tolerated; no clinically meaningful reductions in LVEF or LVEF <50% On track to deliver full 12-week Part D readout in 2Q 2026 and Phase 3 start in 4Q 2026 BOULDER, Colo. , Dec. 24, 2025 /PRNewswire/ -- Edgewise Therapeutics, Inc ., (Nasdaq: EWTX), today announced positive updates from the ongoing CIRRUS-HCM, Phase 2 clinical... Read More