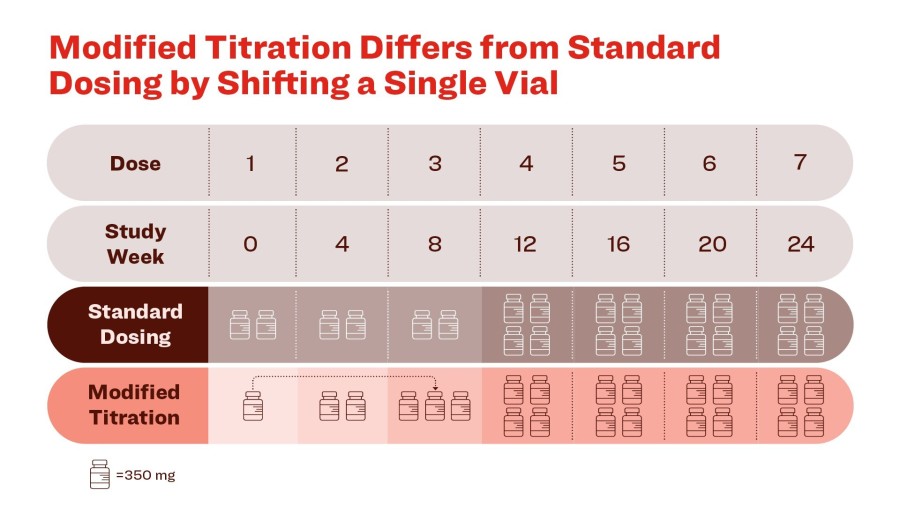
In ATTAIN-MAINTAIN, orforglipron achieved the primary and all key secondary endpoints for weight maintenance vs. placebo at 52 weeks following weight loss on Wegovy or Zepbound Participants who switched to orforglipron from Wegovy maintained all but 0.9 kg of their previously achieved weight loss on average Lilly has submitted orforglipron to the U.S. Food and Drug Administration for the treatment of obesity INDIANAPOLIS ,... Read More