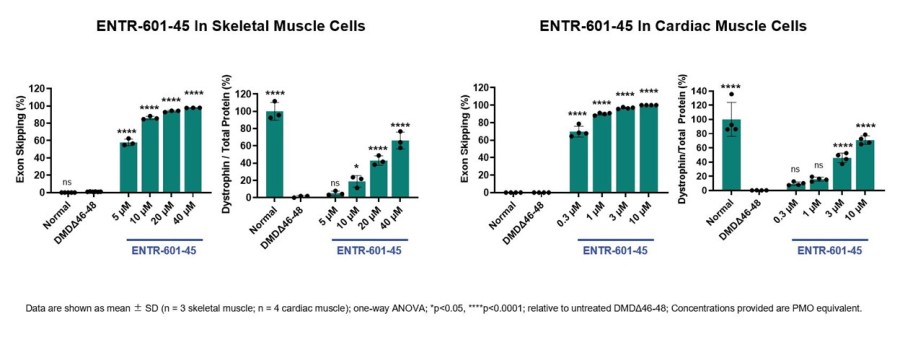
Company on track to report ELEVATE-44-201 data from first patient cohort in Q2 2026 First patient dosed in ELEVATE-45-201 and the Company is on track to report data from the first patient cohort in mid-2026 Filed for regulatory authorization in U.K. to initiate ELEVATE-50-201, a global Phase 1/2 MAD clinical study of ENTR-601-50 Expected cash runway extended into Q3 2027 with $327 million in cash, cash equivalents and... Read More