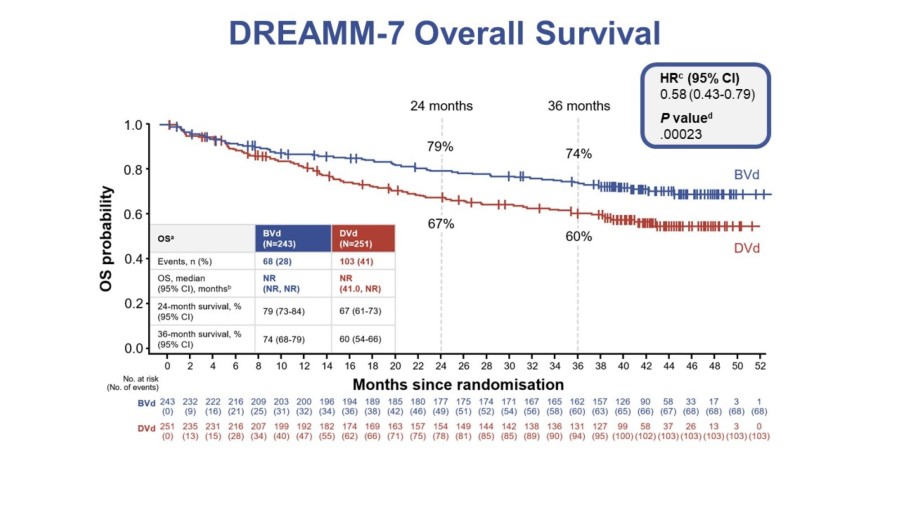
Significant unmet need for patients requires new and novel treatments 1 DREAMM-7 showed a 51% reduction in the risk of death and tripled median progression-free survival in 3L+ indicated population versus a daratumumab-based triplet 2 Blenrep is the only anti-BCMA accessible in the community setting where 70% of patients receive care, and with a new streamlined REMS program 3 Robust clinical development is ongoing to advance... Read More