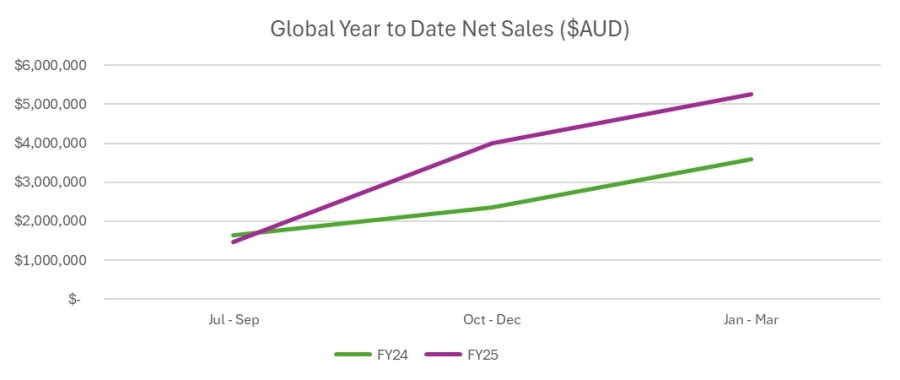
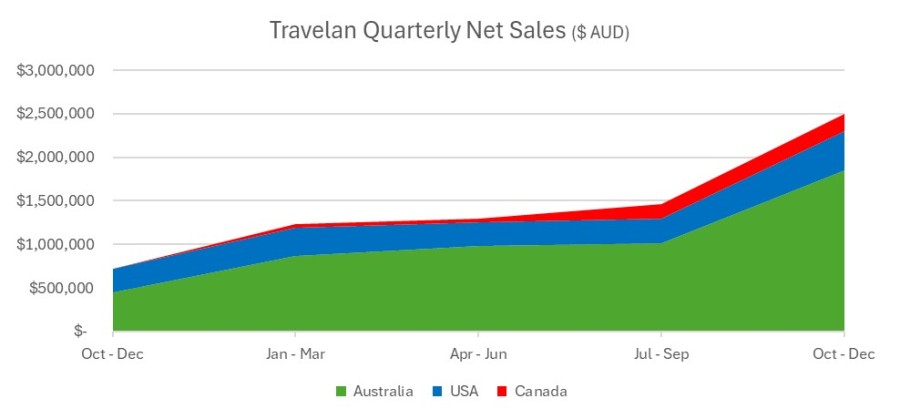
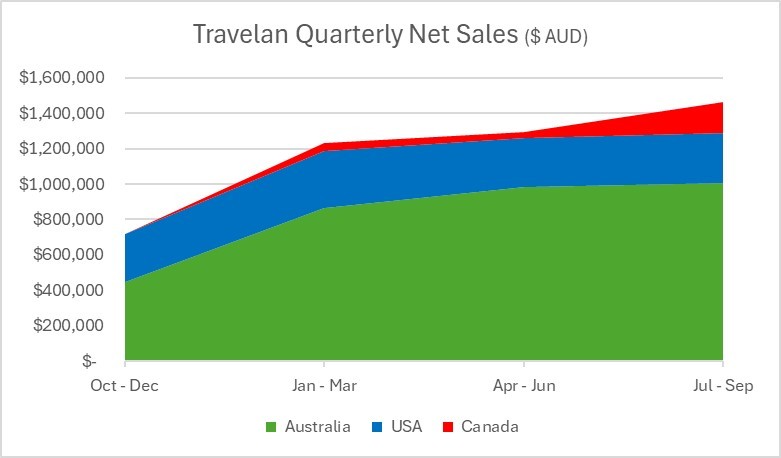
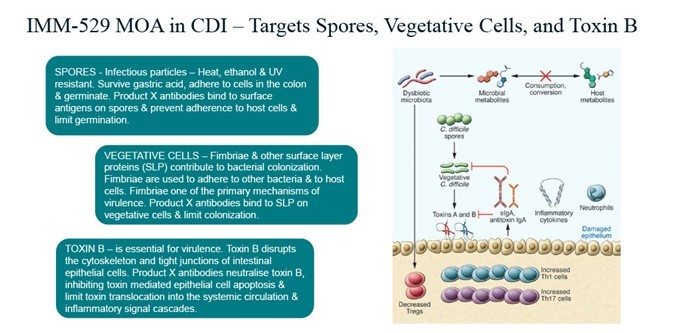
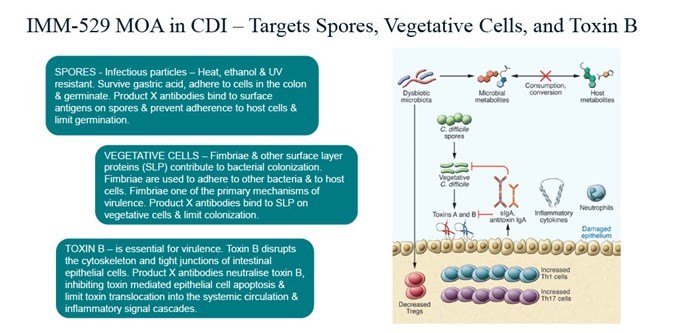
Key Points Immuron receives U.S. Food and Drug administration (FDA) approval for IMM-529 Investigational New Drug (IND) application and clinical study may proceed FDA assigned an IND number (032095) for the IMM-529 application IND 32095 is Immuron’s Investigational new drug (IND) application for clinical development of IMM-529 as product to specifically prevent or treat Clostridioides difficile infection (CDI) and is now... Read More