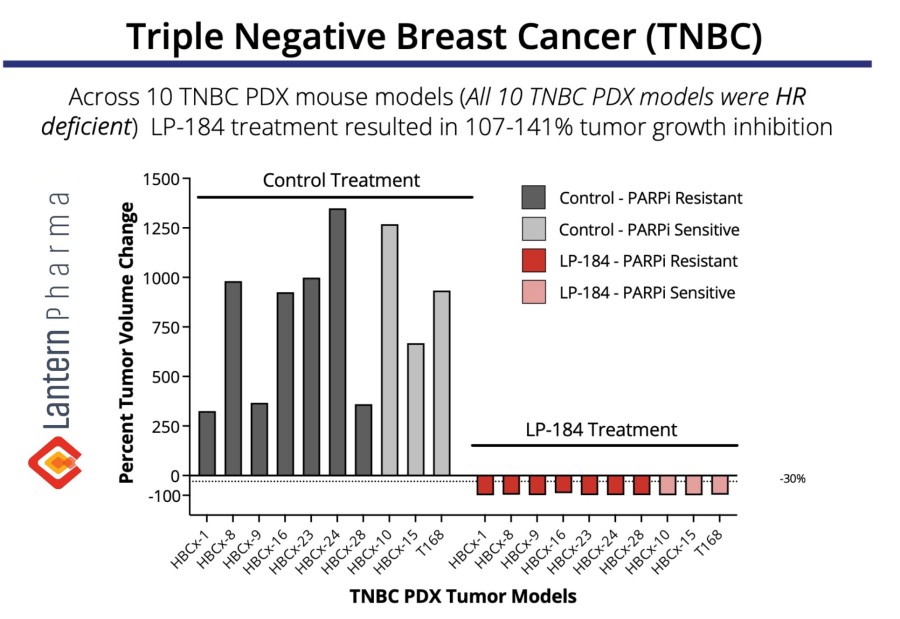
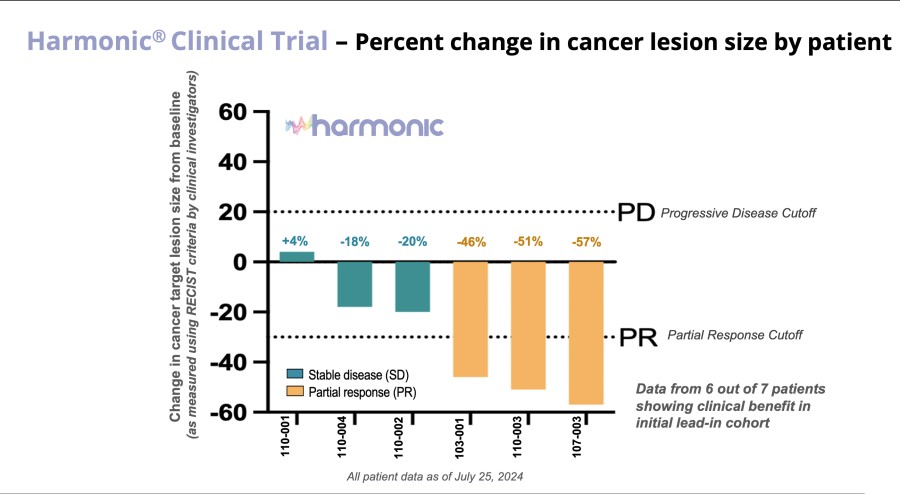
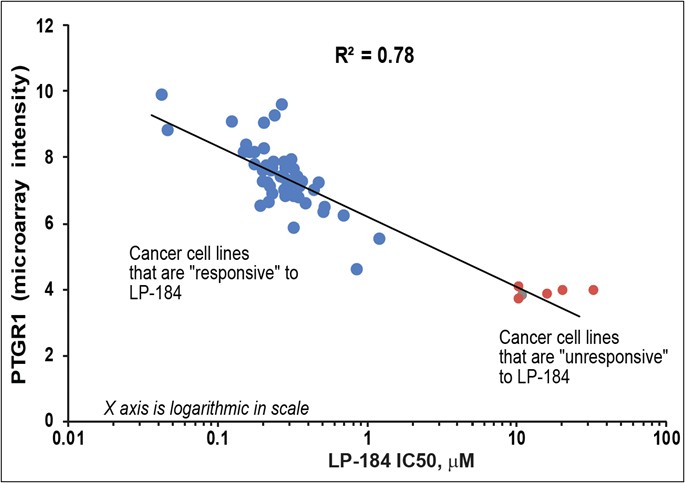
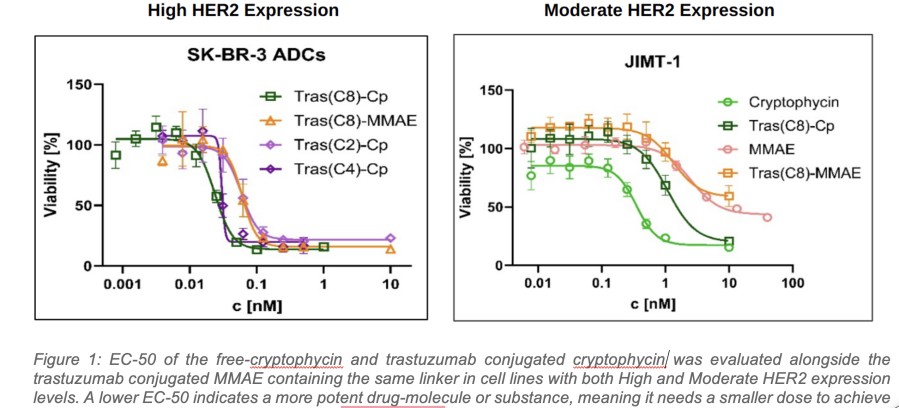
Biomarker-activated cancer drug-candidate LP-184 demonstrates encouraging efficacy signals in DNA damage repair deficient tumors with an acceptable safety and tolerability profile – meeting all primary endpoints. LP-184 demonstrated clinical benefit in multiple highly aggressive cancers with a 54% disease control rate at or above therapeutic dose levels. Multiple Phase 1b/2 clinical trials are now being planned across cancer... Read More