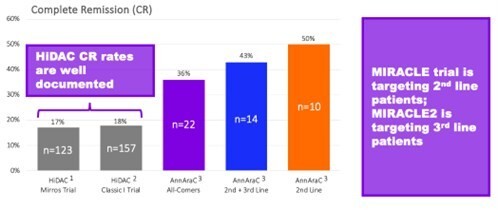
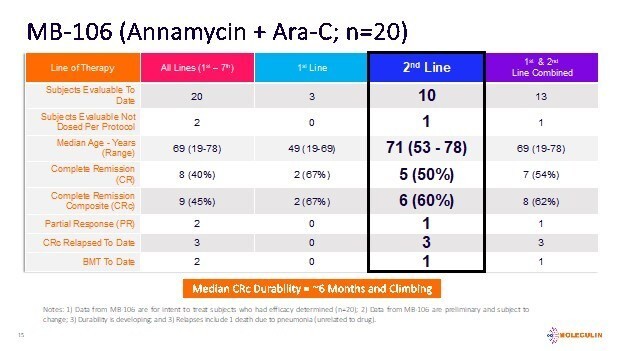
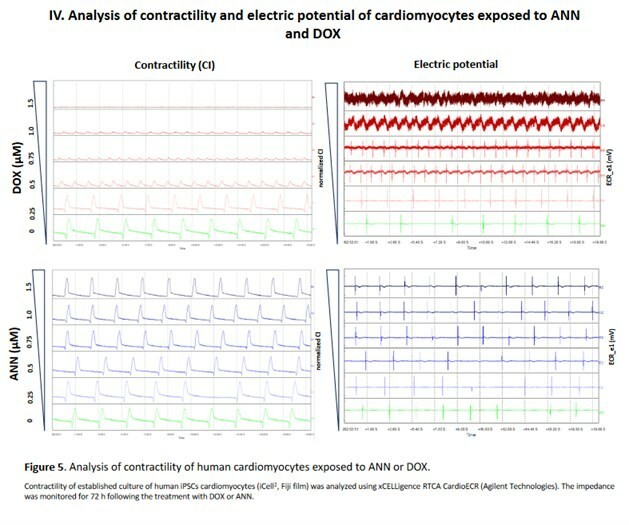
Emory University physician-sponsored clinical trial conducted at the Aflac Cancer and Blood Disorders Center at Children’s Healthcare of Atlanta Results demonstrated WP1066 induces anti-tumor immune responses and were recently published in the Journal of Clinical Investigation Insight WP1066 found to be safe and effective, warranting a Phase 2 trial HOUSTON, Dec. 17, 2025 (GLOBE NEWSWIRE) -- Moleculin Biotech, Inc. ,... Read More