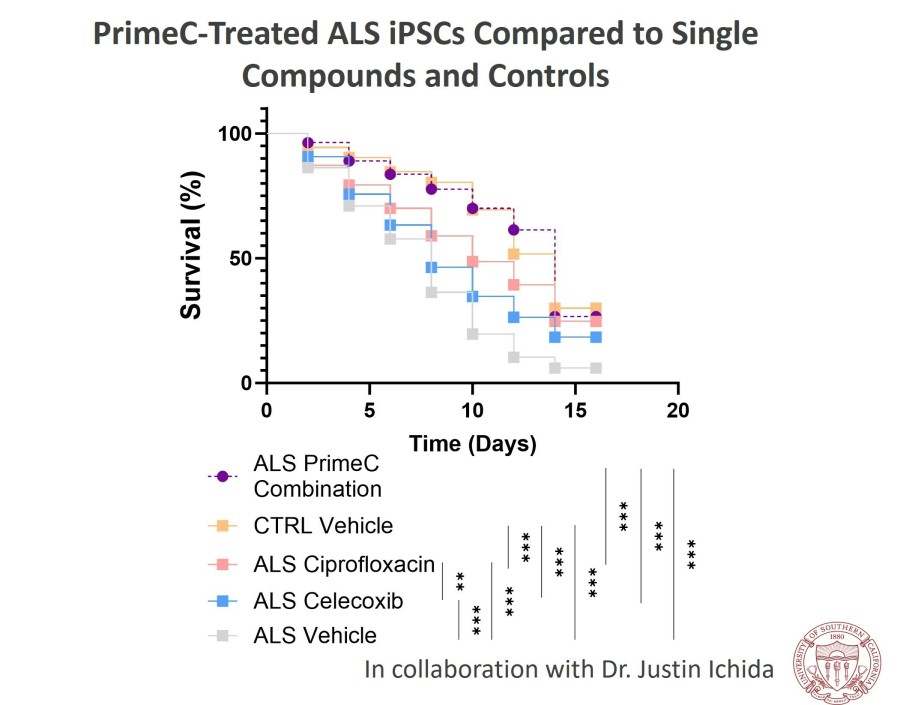
CAMBRIDGE, Mass. , Nov. 24, 2025 /PRNewswire/ -- NeuroSense Therapeutics Ltd. (Nasdaq: NRSN) ("NeuroSense"), a late-clinical stage biotechnology company developing novel treatments for severe neurodegenerative diseases, today announced that the U.S. Food and Drug Administration (FDA) has completed the review of the Investigational New Drug (IND) amendment application and authorized the Company to initiate the pivotal Phase 3... Read More