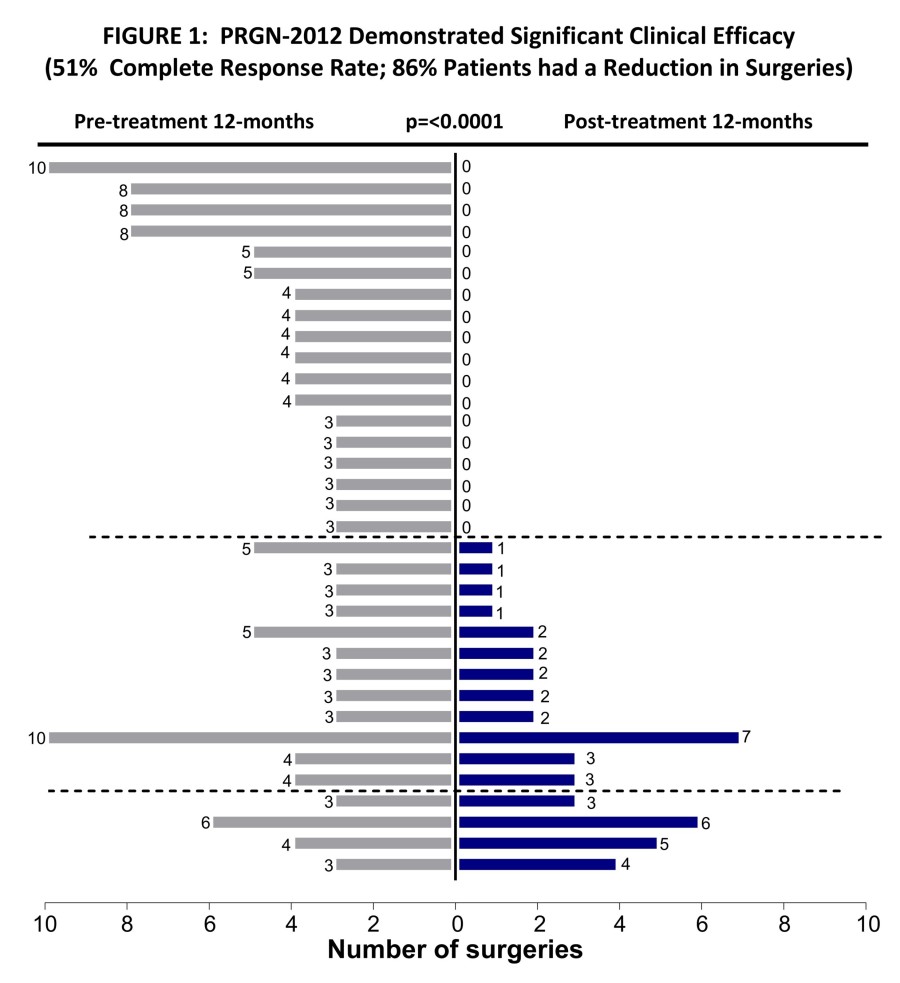
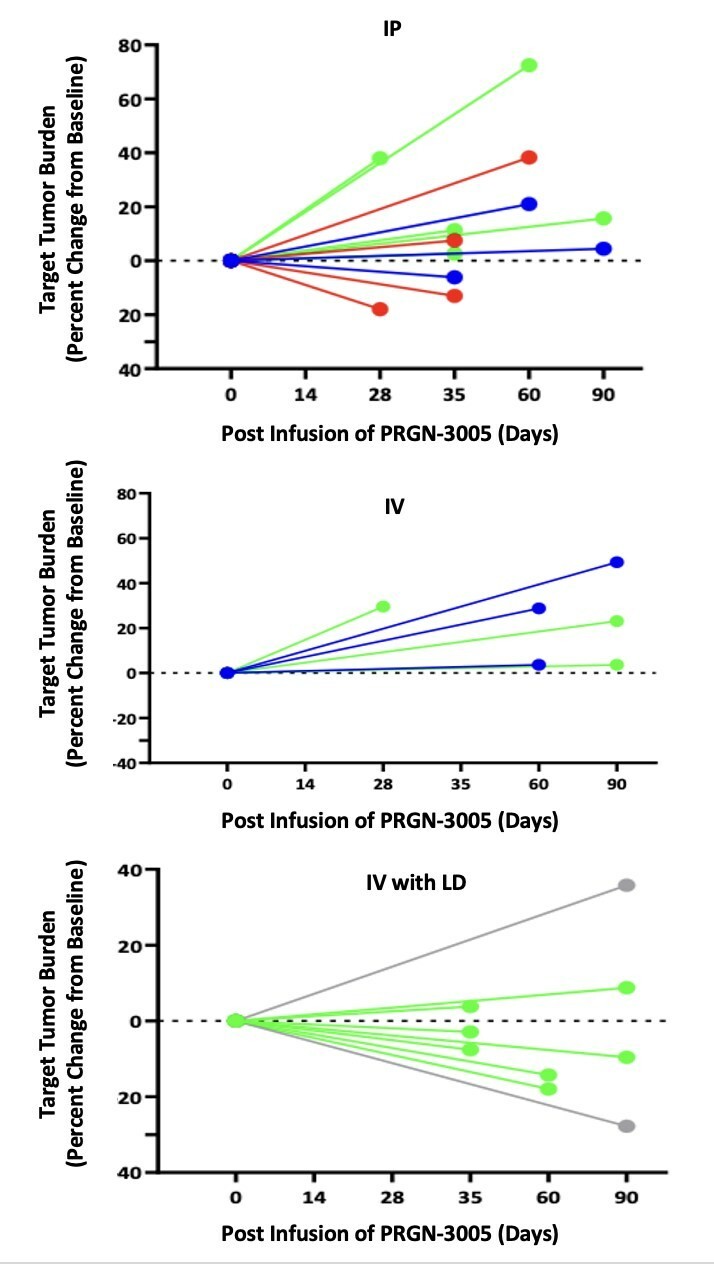
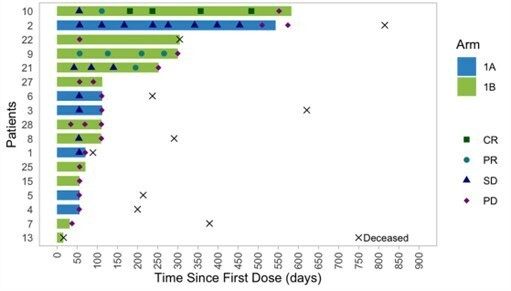
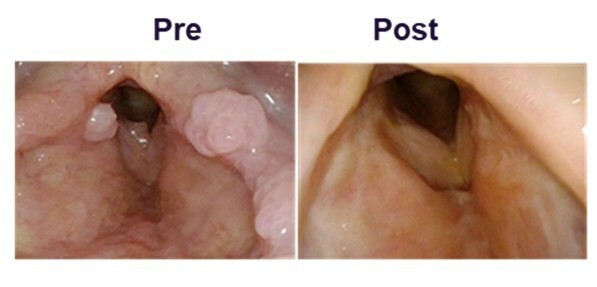
PAPZIMEOS (zopapogene imadenovec-drba) received full approval by the FDA in August PAPZIMEOS launched with a broad label in the US as the first and only FDA-approved treatment for adults with RRP PAPZIMEOS is now available and shipping to prescribers in the US for the treatment of adults with RRP To date, over 100 patients have been registered in the PAPZIMEOS Patient Hub The Company has made significant progress with... Read More