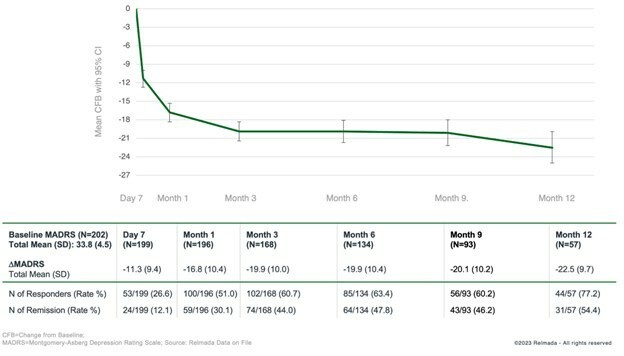
Positive 9-month follow-up data for NDV-01 showed a 92% overall response rate at any time in non-muscle invasive bladder cancer (NMIBC), with favorable overall safety Secured FDA alignment on key elements of Phase 3 program with two independent paths for approval in two separate NMIBC indications: High-risk 2 nd line BCG-unresponsive, and Intermediate-risk patients in the adjuvant setting; Studies expected to begin H1 2026... Read More