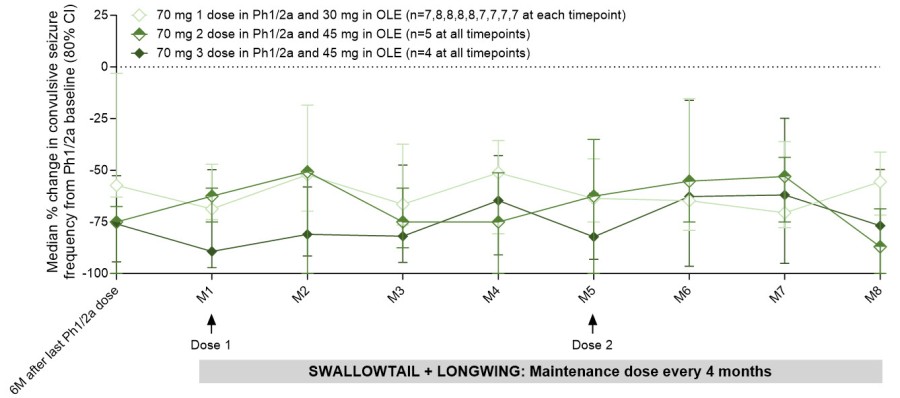
New analyses from the ongoing open label extension (OLE) studies and findings from electroencephalogram (EEG) assessments in patients with Dravet syndrome treated with zorevunersen support the potential for disease modification BEDFORD, Mass. & CAMBRIDGE, Mass. / Dec 01, 2025 / Business Wire / Stoke Therapeutics, Inc. (Nasdaq: STOK), a biotechnology company dedicated to restoring protein expression by harnessing the body’s... Read More