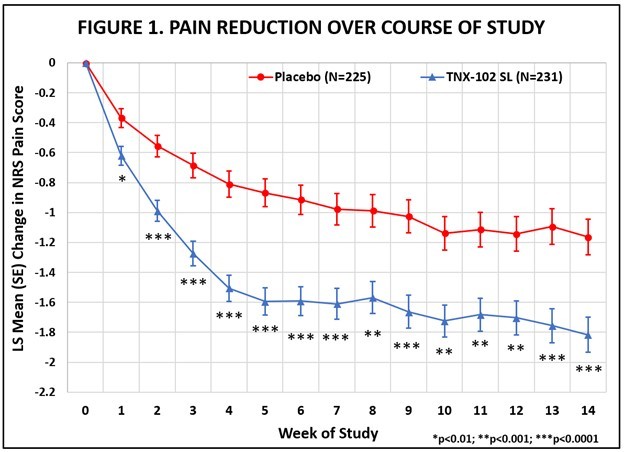
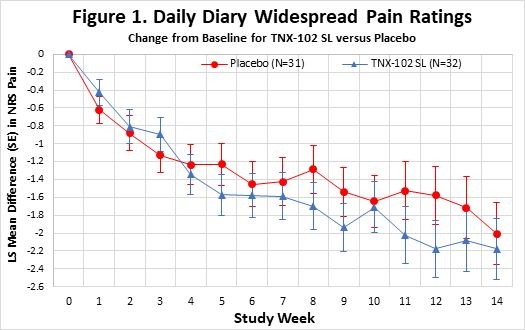
Non-opioid analgesic shows efficacy in several animal pain models, including diabetic and chemotherapy-induced neuropathic pain Compelling safety and pharmacokinetic profiles in animals support IND-enabling studies CHATHAM, N.J., Dec. 16, 2025 (GLOBE NEWSWIRE) -- Tonix Pharmaceuticals Holding Corp. (Nasdaq: TNXP) (“Tonix” or the “Company”), a fully-integrated commercial biotechnology company, today announced licensing... Read More