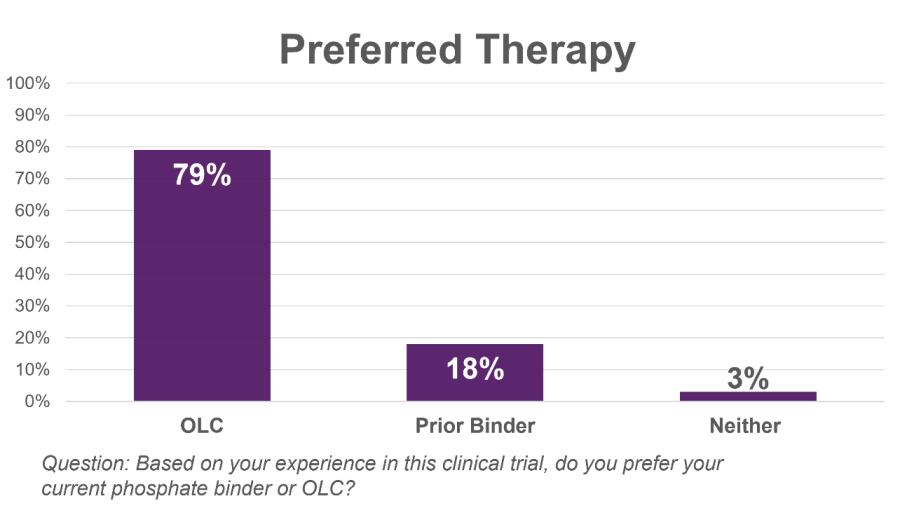
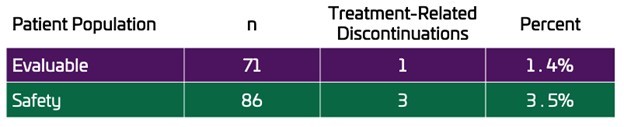
LOS ALTOS, Calif., Nov. 25, 2025 (GLOBE NEWSWIRE) -- Unicycive Therapeutics, Inc. (Nasdaq: UNCY), a clinical-stage biotechnology company developing therapies for patients with kidney disease (the “Company” or “Unicycive”), today announced that Shalabh Gupta, M.D., Chief Executive Officer, will participate in two investor events in December. Event: Piper Sandler 37th Annual Healthcare Conference Type: Fireside chat Date/Time:... Read More