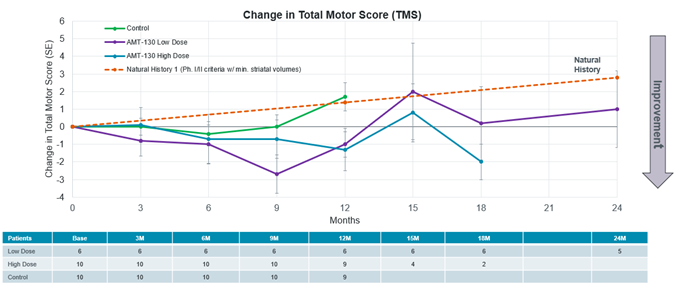
Announced pivotal topline data from Phase I/II study of AMT-130 in Huntington’s disease met its primary and key secondary endpoints, demonstrating statistically significant slowing of disease progression at 36 months and supportive trends across key clinical and biomarker endpoints Preliminary feedback from FDA at a recent pre-Biologics License Application (BLA) meeting for AMT-130 indicated a key shift from prior regulatory... Read More